Alkali Type Spectra and Equivalent Electrons in Physics
Alkali-type spectra arise from atoms with a single outer electron—like Li, Na, and K—producing sharp, simple emission and absorption lines. These transitions help identify equivalent electrons and reveal atomic structure, energy levels, and electron configurations, with major applications in spectroscopy, astrophysics, and quantum mechanics.

Equivalent Electrons and Alkali Type Spectra
- The emission or absorption spectra of alkali metals, like lithium, sodium, potassium, rubidium, and caesium, are called alkali type spectra.
- These metals are in Group 1 of the periodic table.
- There is only one electron in the top shell of these metals, which makes their spectral lines look different when energy changes.
Characteristics
- Simple Spectra: The atoms in alkali metals aren't very complicated, so their spectra have fewer lines than those of metals with more complicated atoms.
- Sharp Lines: The spectral lines that are made are clear and sharp, which makes them easier to study.
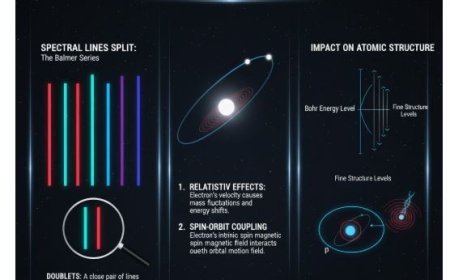
- Lines: Because there is only one outer electron, alkali spectra have a number of lines that show the different electronic changes.
Importance
- It's important to understand atomic structure and electron shifts by looking at alkali type spectra.
- Figuring out what elements are in many areas, like astrophysics and chemistry.
2. The Atom and How It Is Put Together
A Look at Atoms
- The smallest piece of matter that still has the characteristics of an element is called an atom. It has a centre and electrons all around it.
How Atoms Are Put Together
- The nucleus is made up of neutrons and protons.
- Electrons are negatively charged particles that move in a circle around the nucleus at set amounts of energy.
3. How Electrons Are Arranged in Alkali Metals
Electron Configuration
- The arrangement of electrons in an atom's energy levels and orbitals is called its electron configuration.
Configuration for the Ground State
- When an alkali metal is in its ground state, the electron on the outside has the most energy (n=1 for Li, n=2 for Na, etc.).
Examples:
- Lithium (Li): 1s² 2s¹
- Sodium (Na): 1s² 2s² 2p⁶ 3s¹
Configuration of an Excited State
- When you add energy to an atom, its electrons can jump to higher energy levels, making the atom excited.
- Example: If an atom of sodium takes in energy:
- Ground state: 1s² 2s² 2p⁶ 3s¹
- Excited state: 1s² 2s² 2p⁶ 3s⁰ 3p¹
4. Learn About Equivalent Electrons
What Equivalent Electrons Mean
- Equivalent electrons are electrons that have the same energy amounts and shapes and exist in the same place or state.
- In alkali metals, the electron at the very edge is equivalent to other electrons in the same shell.
What Role Do Equivalent Electrons Play in Spectra?
- The outer electron creates the spectral lines seen in alkali type spectra because its transitions cause light to be emitted or absorbed.
- Each equivalent electron contributes to a different spectral line based on the transitions it can go through.
How to Figure Out Equivalent Electrons
- Look at the valence shell to find the number of equivalent electrons:
- Sodium (Na): One equivalent electron is in the 3s orbital.
- This idea helps figure out transition possibilities and, by extension, the spectral lines.
5. How to Use Alkali Type Spectra in Spectroscopy
- Spectroscopy is the study of how matter interacts with electromagnetic energy.
- Alkali spectra are used to identify elements by looking at the unique line emissions they give off.
Applications of Alkali Spectra
- Astrophysics:
- Used in astrophysics to determine the composition of stars and celestial objects by analyzing their light emissions and absorptions.
- Quantum Mechanics:
- Helps in understanding atomic transitions, energy levels, and fundamental principles like quantization in quantum mechanics.
What's Your Reaction?