The Raman Effect: Classical and Quantum Theories
Explore the Raman Effect—an inelastic light-scattering phenomenon discovered by C.V. Raman that reveals molecular vibrations and structure. Learn its classical and quantum explanations, including Stokes and Anti-Stokes lines, and how Raman spectroscopy supports chemical analysis, biology, and material science.
The Raman Effect: Classical and Quantum Theories
- In physics, the Raman Effect is a very interesting effect that has big effects in many areas, including spectroscopy, chemistry, and material science.
- Indian scientist C.V. Raman was the first person to describe this effect in 1928. It helps us understand molecular shapes and vibrations.
The Raman Effect
- When light reacts with molecules, its wavelength (or colour) changes. This is called the Raman Effect.
- Most of the light that goes through a medium is scattered in an elastic way, keeping the same wavelength.
- However, a small amount of light is scattered in an inelastic way, changing the wavelength. The vibrational states of molecules can be learnt from this shift.
- Important things about the Raman Effect:
- 1.When there is inelastic scattering, the wavelength changes. This is different from Rayleigh scattering, where the wavelength stays the same.
- Energy Exchange: The change in wavelength is equal to the energy changes that happen when molecules move or vibrate.
- Techniques That Work Well Together: Infrared spectroscopy and Raman spectroscopy are often used together to get a more complete picture.
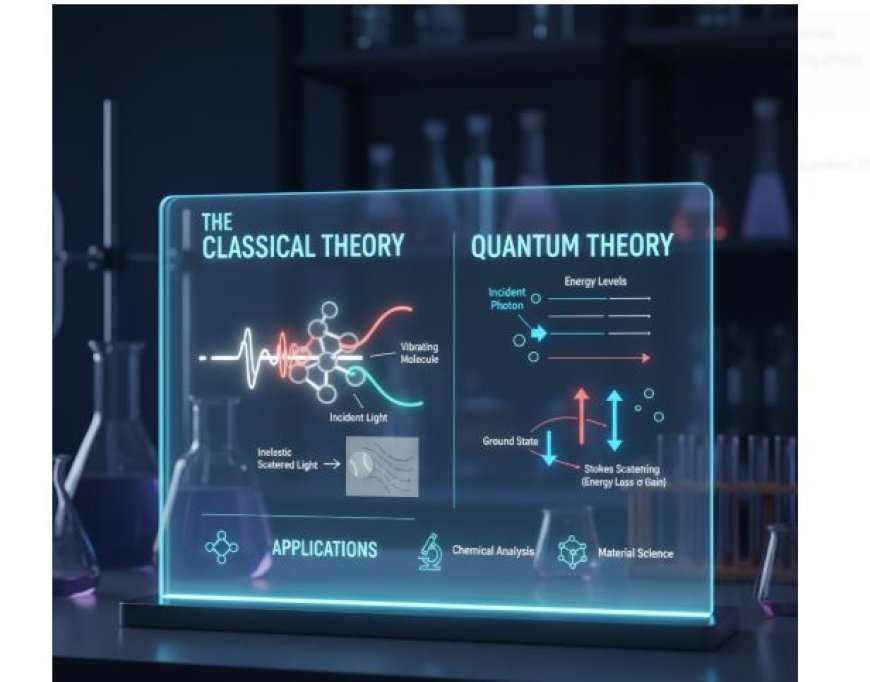
The Raman Effect from a Classical Point of View
- Electromagnetism and molecular movements can be used to understand the Raman Effect from a traditional point of view.
Key Points of the Classical Theory:
- Electromagnetic Waves: Light looks like an electromagnetic wave that moves through molecules.
- Pollarizability: When an electromagnetic wave hits a molecule, it can cause a dipole moment because the electrons move around. "Polarisability" is the word for this result.
- Vibrating Modes: Molecules have different vibrating modes, which cause links to stretch and bend.
- Light reacts with these modes and either takes in or gives off energy, which changes the colour.
Diagrammatic Representation:
Think about how light hitting a molecule is like a tennis ball hitting a wall.
Vibrates means that the wall moves a little, which changes the direction (wavelength) of the ball (light) after it bounces.
The Raman Effect from a Quantum Point of View
Using the rules of quantum physics, the quantum theory of the Raman Effect looks at things in a more basic way.
Key Points of the Quantum Theory:
- Photons and Levels of Energy: Photons are the particles that make up light. There are different levels of quantised energy in molecules, and photons can change these values.
- Lines of Stokes and Anti-Stokes: Based on differences in energy, the Raman Effect can be broken down into two groups:
- Stokes Scattering: This happens when photons lose energy (lower frequency) after reacting with molecules that are moving back and forth.
- Anti-Stokes Scattering: This happens when photons get more energy from the vibrating modes and travel at a higher frequency.
Energy Chart:
- In quantum mechanics, think about molecules that have different amounts of energy. If photons engage with molecules, they can either raise the energy level of the molecules (Stokes) or let the energy be passed back to the photons (Anti-Stokes).
Applications of the Raman Effect
The Raman Effect can be used in many real-world situations, including:
- Chemical Analysis: Raman spectroscopy is used a lot to figure out the shapes of molecules and the chemicals that make them up.
- Biological Studies: It helps look at biological parts, figure out what proteins are, and learn about how cells work.
- In material science, the Raman Effect is used to describe new materials, plastics, and nanomaterials.
What's Your Reaction?