Pauli's Exclusion Principle and Its Physical Significance on the Periodic Table
Discover Pauli’s Exclusion Principle and how it shapes atomic structure, electron configuration, and the periodic table. Learn its physical significance in chemistry, magnetism, and material properties.
Pauli's Exclusion Principle and Its Physical Significance on the Periodic Table
Physics and chemistry are inextricably linked, particularly when it comes to the organisation of elements in the periodic table. Pauli's Exclusion Principle and its consequences are some of the fundamental notions that govern this thinking.
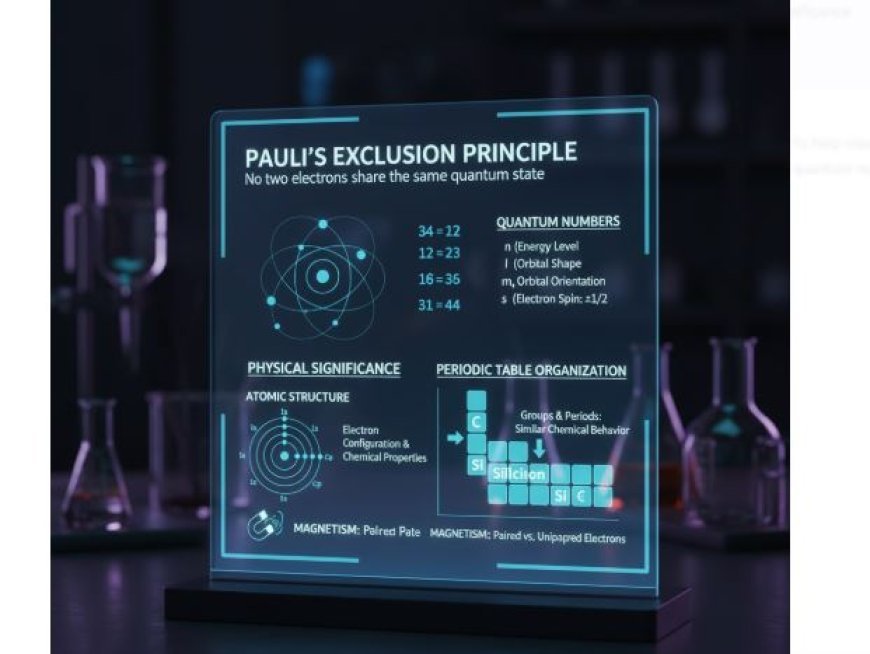
Pauli’s Exclusion Principle
The Pauli Exclusion Principle, first suggested by scientist Wolfgang Pauli in 1925, is a key idea in quantum mechanics. It says that:
- Fermions, such as electrons, protons, and neutrons, cannot share the same quantum state. The primary focus here is on electrons within an atom.
- The principle is summarised as follows:
• Each electron in an atom requires an own set of quantum numbers. These statistics define an electron's energy, shape, and orientation within an atom.
Quantum Numbers
- To further grasp this principle, let's briefly discuss quantum numbers:
- The Principal Quantum Number (n) indicates an electron's energy level (1, 2, 3, etc.).
- The azimuthal quantum number (l) indicates the shape of the orbital (e.g., s, p, d, f).
- The Magnetic Quantum Number (m) indicates the orientation of an orbital in space.
- The Spin Quantum Number (s) indicates the direction of an electron's spin. An electron can spin up (+1/2) or down (-1/2).
- Because each set of quantum numbers must be unique, numerous electrons in an atom cannot have the same values for all four quantum numbers. This is critical for understanding the structure of atoms.
The Physical Significance of Pauli's Exclusion Principle
The consequences of Pauli's Exclusion Principle go beyond quantum mechanics. Here's how it affects several physical phenomena and the arrangement of the periodic table:
1. Structure of Atoms
-
- Electron configuration: The configuration of electrons in an atom is determined by this concept.
- Because no two electrons may have the same set of quantum numbers, atoms arrange their orbitals in a certain order.
- Chemical Properties: An element's electron arrangement directly affects its chemical interactions with others.
- Elements with similar configurations will behave chemically similarly.
2. Stability of Matter
-
- Orbital Formation: Electrons form stable orbitals around a nucleus by filling them from the lowest energy levels (1s upwards).
- Atomic size is determined by the arrangement of electron shells. Electrons located further from the nucleus have greater energy levels, and their configurations define the atomic radius.
3. Periodic Table Organization
-
- Elements are arranged in the periodic table based on their electron configurations, affected by the Exclusion Principle. For example:
- Groups and Periods: Elements are classified into columns based on their electron configurations, resulting in comparable chemical characteristics.
- Atomic Number and Energy Levels: Moving down a group in the periodic table adds more electron shells, which can affect chemical reactivity and physical attributes.
- Magnetism & Material Properties
-
- Electron arrangement impacts a material's interaction with magnetic fields, known as paramagnetism and diamagnetism. Atoms containing unpaired electrons (as per Pauli's
- Exclusion Principle) display paramagnetism, while those with all paired electrons are often diamagnetic.
IMAGE SOURCE (THUMBNAIL)
What's Your Reaction?