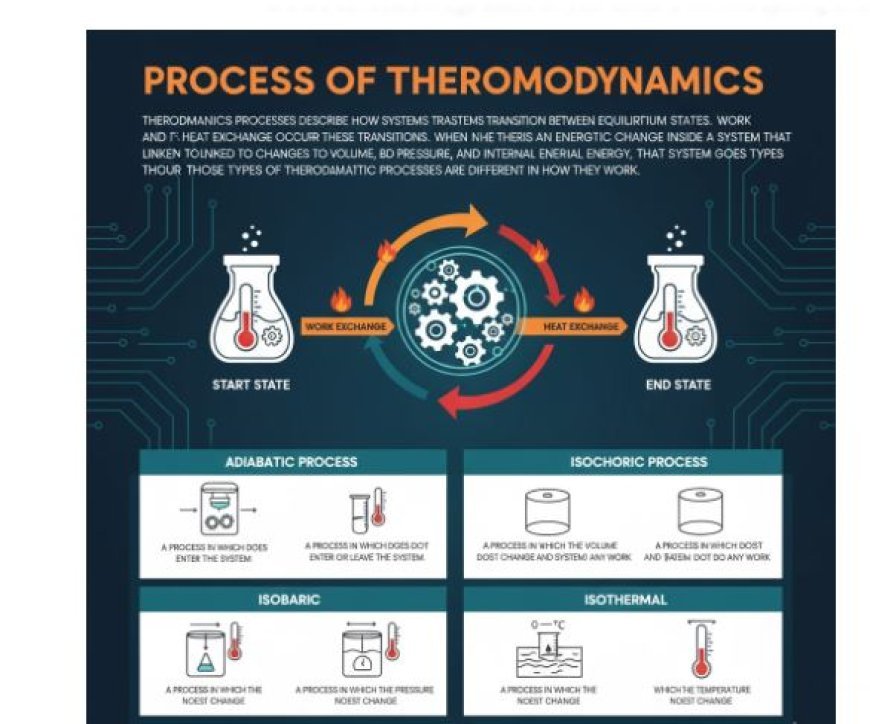
PROCESS OF THERMODYNAMICS
Thermodynamics processes describe how systems transition between equilibrium states. Work and heat exchange occur during these transitions.
Thermodynamics Process
- When there is an energetic change inside a system that is linked to changes in pressure, volume, and internal energy, that system goes through a thermodynamic process.
- All four of these types of thermodynamic processes are different in how they work.
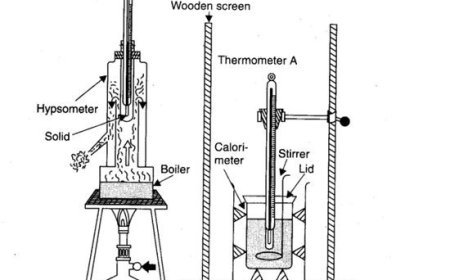
Adiabatic Process
- A process in which heat doesn't enter or leave the machine.
Isochoric Process
- A process in which the amount doesn't change and the machine doesn't do any work.
Isobaric Process
- A process in which the pressure doesn't change.
Isothermal Process
- A process in which the temperature doesn't change.
What's Your Reaction?