KINETIC THEORY OF GASES
Kinetic theory of gases: Imagine tiny particles in constant motion, explaining gas pressure and behavior.
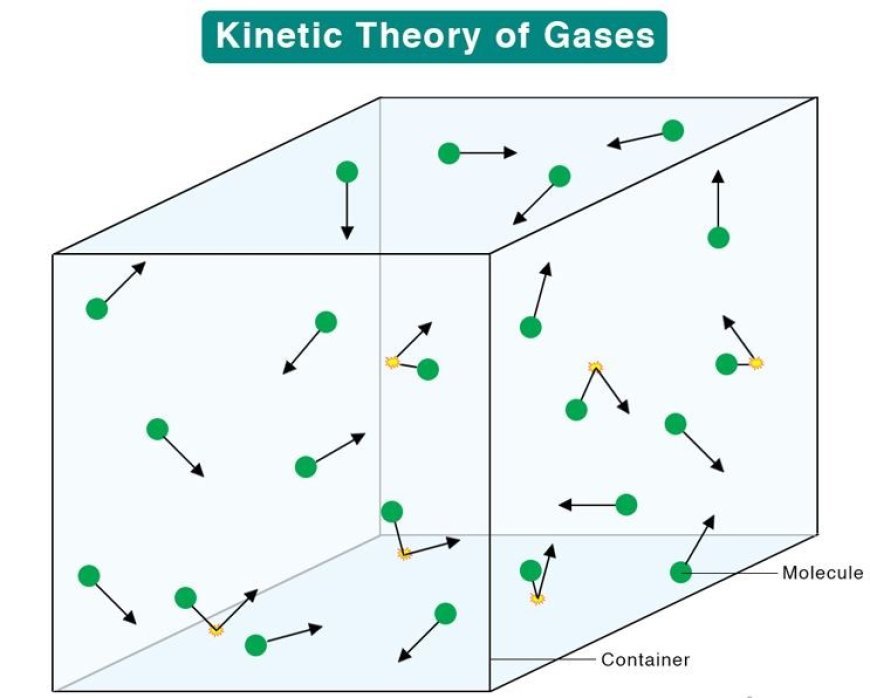
Kinetic Theory of Gases
- In the 1800s, scientists James Clark Maxwell, Rudolph, and Clausius came up with the kinetic theory of gases to explain how gases behave.
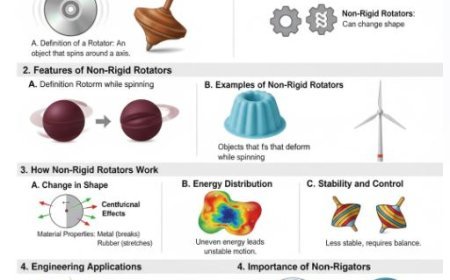
- The idea says that gas is made up of very small, hard spheres that move around and interact with the wall's surface.
- The circles show the molecules of gas, and they move based on Newton's rules of motion, which were created in the 1600s.
- It talks about how molecules change things about gases, like their temperature and pressure. It also tells us why Boyle's law works for gases.
- We now know that the following equation shows how the pressure (P), volume (V), and temperature (T) of gases at low temperatures are related:
- Where n is the number of moles in the gas and R is the gas constant, which is 8.314 JK-1mol-1.
- Any gas that fits this equation is now known as an ideal gas. This is why the equation is called the ideal gas equation.
- But there are some assumptions we make when we talk about how perfect gases behave.
Assumptions of the Kinetic Theory of Gases
There are some factors that support the kinetic theory of gases.
- Molecules in all gases are always and consistently moving in different directions.
- The molecules are far apart, even though they are the same size.
- When a gas sample is kept in a container, the molecules of the sample don't push against the walls of the container when they hit each other.
- A collision between two molecules and a clash between a molecule and the wall are thought to happen very quickly.
- This means that all collisions between molecules and even between molecules and walls are thought to be fluid.
- If you take a sample of a certain gas, all the molecules in it follow the rules of motion.
- When a gas sample is left alone for long enough, it will reach a steady state.
- It doesn't matter where, how far, or when molecules are; their quantity and spread don't change.
What's Your Reaction?