Huckel's Molecular Approximation
Hückel’s Molecular Approximation is a simplified method for analyzing π-electron systems in conjugated molecules like benzene. It uses molecular orbital theory and LCAO to predict energy levels, aromaticity, and electronic structure, helping explain stability, reactivity, and UV-Vis properties. Though ideal for planar cyclic molecules, it remains a foundational tool in theoretical and computational chemistry.
Huckel's Molecular Approximation
Huckel's Molecular Approximation is a helpful tool in theoretical chemistry and physics to explore the electronic structure of conjugated molecules. This reduction enables scientists to better understand how electrons interact in these compounds and anticipate their features.
Introduction to Huckel's Molecular Approximation
- Huckel's molecular approximation focuses mostly on simple aromatic molecules such as benzene.
- It aids in analyzing π (pi) electron systems, which are essential for understanding the stability and reactivity of organic compounds.
Key Concepts
- Conjugation involves alternating single and double bonds in a molecule. Conjugated systems allow electrons to be shared by several atoms, which increases stability.
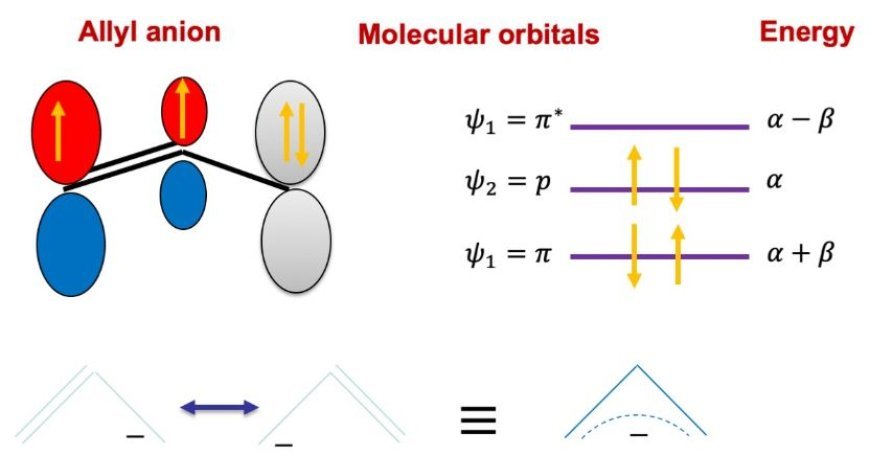
- π Electrons: These electrons occupy the pi molecular orbitals, which are generated by overlapping p-orbitals. Conjugated systems' chemical characteristics depend heavily on π electrons.
- Huckel's Approximation is based on molecular orbital theory, which describes electron behavior in molecules.
Basics of Huckel's Molecular Approximation
Huckel's molecular approximation simplifies difficult calculations for determining the energy levels and distributions of π electrons. Here are the key principles:
1. Simple Model of π Electrons
- Huckel's approximation considers just π electrons in computations, neglecting complicated interactions with sigma (σ) electrons.
- The approximation reduces the molecule to a graph, with each atom as a vertex and bonds as edges.
2. Linear Combination of Atomic Orbitals (LCAO)
Huckel postulated that molecular orbitals are a combination of atomic p-orbitals.
- The equation is:
where ψ is the molecular orbital, φ represents the atomic orbitals, and c are the coefficients.
3. Energy Levels and the Eigenvalue Problem
- Huckel's approximation creates an eigenvalue issue, which identifies energy levels associated with π electrons.
- The characteristic polynomial, known as the Huckel equation, may be represented as:
- Here, α denotes the energy of the atomic orbitals and β indicates the interaction between them.
4. Molecular Orbitals and Electron Configuration
- Using the Huckel equation, one may anticipate the energy levels of molecular orbitals and the number of π electrons they will contain.
- The Pauli exclusion principle states that each orbital can carry two electrons with opposing spins.
Applications of Huckel's Molecular Approximation
Huckel's molecular approximation has a number of useful applications in chemistry and physics:
1. Understanding Aromaticity
- The approximation determines whether a compound is aromatic (stable and cyclic), anti-aromatic (unstable), or non-aromatic based on calculated energy levels.
- Aromatic compounds adhere to Hückel's rule, which states that a planar, cyclic molecule is aromatic if it has (4n + 2) π electrons, where n is a non-negative integer.
2. Predicting Molecular Characteristics
- Huckel's approximation predicts physical characteristics including bond lengths, angles, and reactivity of conjugated systems.
- It also helps explain UV-Vis absorption spectra, which are crucial for characterizing organic molecules.
3. Computational Chemistry
- Huckel's approximation is the foundation for advanced computational approaches in quantum chemistry and molecular modeling.
Limitations of Huckel's Molecular Approximation
While Huckel's approximation is a great tool, it does have certain limits:
1. Simplistic Assumptions
- The model's simplicity may miss critical electron interactions, especially in complicated compounds with many substituents.
2. Applicability
- It works well for planar, cyclic structures and may not adequately reflect three-dimensional conformations of molecules.
What's Your Reaction?