ROTATIONAL SPECTRA OF DIATOMIC MOLECULES
Rotational spectra of diatomic molecules arise from quantized rotational energy levels, where molecules absorb or emit microwave radiation during transitions between states defined by quantum number J. These spectral lines reveal key molecular properties such as bond length, moment of inertia, and atomic masses. Rotational spectroscopy is widely used in chemistry, astronomy, and atmospheric science to identify molecules, analyze structures, and study interstellar and atmospheric compositions.
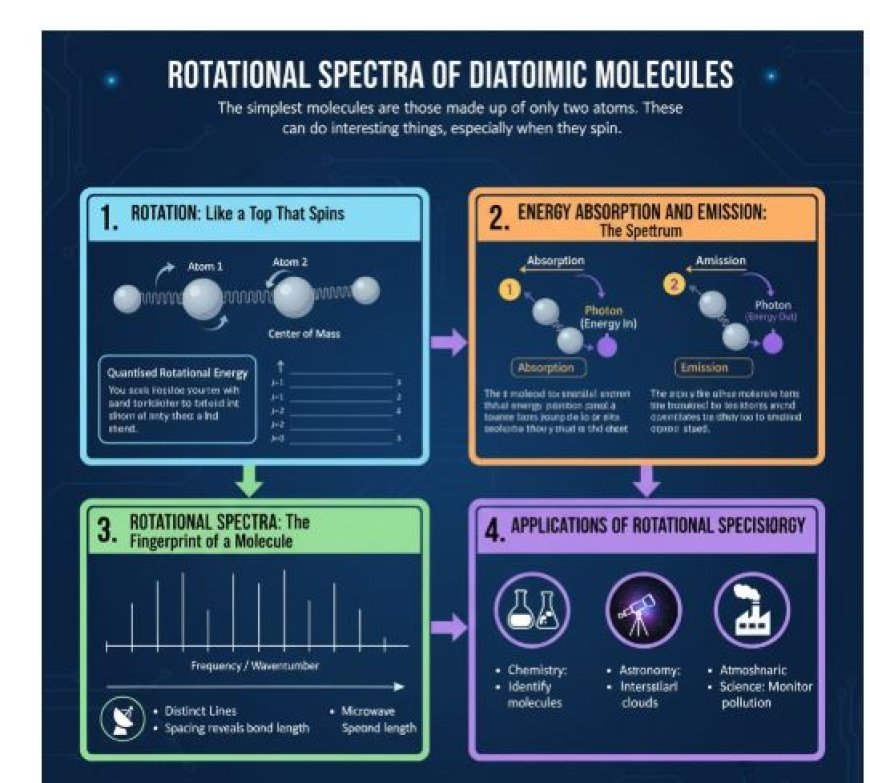
ROTATIONAL SPECTRA OF DIATOMIC MOLECULES
The simplest molecules are those made up of only two atoms, such as oxygen (O₂) and hydrogen chloride (HCl). But these structures, even the ones that look simple, can do interesting things, especially when they spin.
1. Rotation: Like a Top That Spins
A diatomic molecule is like a small dumbbell. Its two atoms are linked by a bond, which acts as the "handle." This dumbbell is rotating when it starts to spin around its centre of mass.
Quantised Rotational Energy:
- A diatomic molecule can only rotate within certain energy levels, unlike a large spinning top.
- In other words, the molecule can only spin at specific speeds that match certain amounts of energy.
- Quantum physics is based on the idea that energy is quantised.
Spinning Energy Levels:
- A molecule's energy is based on its spinning speed and moment of inertia, which is how hard it is to change its rotation.
- Higher energy levels correspond to faster rotating speeds.
- A quantum number, "J," represents these energy levels.
- J can be any value from 0 to 9 (inclusive):
- J = 0 means no spin.
- J = 1 represents the lowest rotational energy level.
- Higher J values correspond to higher rotational energy.
2. Energy Absorption and Emission: The Spectrum
When diatomic molecules transition between rotational energy states, they can either absorb or emit energy.
- A molecule can jump to a higher rotational energy level (higher J number) by absorbing energy from an external source, such as light.
- Emission: When a molecule loses energy, it emits photons, transitioning to a lower rotational energy level with a smaller J number.
3. Rotational Spectra: The Fingerprint of a Molecule
The energy difference between two rotational levels determines the frequency of light a molecule absorbs or emits, creating a unique set of spectral lines called the rotational spectrum.
Different Lines:
- The spectrum displays distinct lines for each rotational transition.
- The spacing between these lines provides insights into the molecule’s bond length and moment of inertia.
- Spinning Energy Levels In the electromagnetic spectrum, rotational transitions typically occur in the microwave region.
- Microwave spectroscopy is a powerful tool for analyzing how diatomic molecules rotate and their structural properties.
What Spectra Tell Us:
Scientists can determine the following from rotational spectra:
- The bond length of a diatomic molecule.
- The molecule's moment of inertia.
- The types of bonds within the molecule.
- The masses of the atoms in the molecule.
4. Applications of Rotational Spectroscopy
Understanding rotational spectra has applications in various scientific fields, including:
Chemistry:
- Identifying unknown molecules.
- Studying molecular shapes.
- Understanding chemical reactions.
Astronomy:
- Detecting rotational spectra of molecules in space.
- Learning about the composition of interstellar clouds and planetary atmospheres.
Atmospheric Science:
- Monitoring air pollution.
- Understanding chemical reactions in the Earth's atmosphere.
What's Your Reaction?