THE HYDROGEN ATOM
Discover how fine structure in the hydrogen atom arises from relativistic effects and spin–orbit coupling, revealing the true quantum nature of atomic energy levels.
THE HYDROGEN ATOM
- It is the simplest atom in the world, but it has taught us a lot about quantum physics.
- The simple Bohr model did a good job of understanding the spectral lines and energy levels of hydrogen.
- The fine structure of hydrogen's spectral lines, on the other hand, shows an interesting amount of complexity when you look more closely.
The Fine Structure
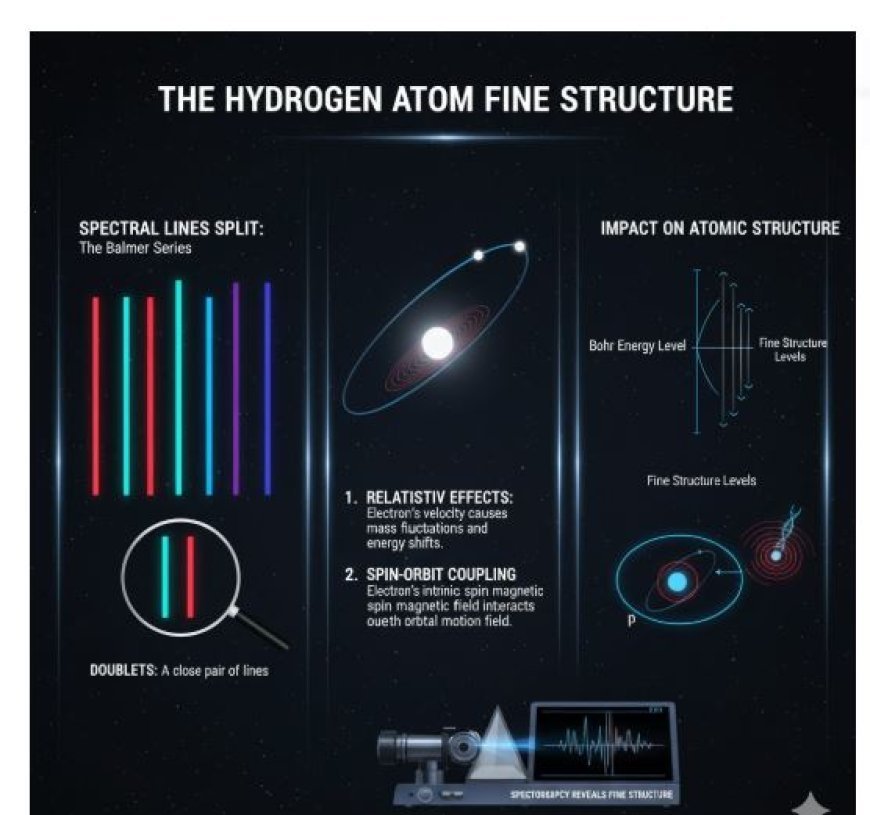
- Think about focussing on just one of the spectral lines that hydrogen gives off. That's right, it's not just one sharp line. It's a group of lines that are very close to each other.
- The "fine structure" is what you call it when spectral lines split in two. Relativity and the way the electron's spin and orbital rotational momentum work together make this happen.
Let's break it down:
1. Relativistic Effects:
- Electron Velocity: The electron in a hydrogen atom moves very slowly around the core. It doesn't stay in one place.
- Einstein's Relativity: According to Einstein's theory of special relativity, an object's mass increases as its speed increases. In other words, the electron's mass changes over time.
- Energy Shift: This change in mass makes a small change to the electron's energy levels, which makes the photon's energy change a little. This change makes the fine structure breaking worse.
2. Spin-Orbit Coupling:
- Spin of an electron: Electrons have a trait called spin that makes them behave like tiny magnets.
- This is another kind of spinning motion. It is made when the electron goes around the nucleus.
- How Magnetic Fields Interact: The electron's spin makes a magnetic field that interacts with the field that its circular motion makes. In physics, this is known as spin-orbit coupling.
- Even more energy levels are split in half by this process, which adds more fine structure lines to the spectrum.
Thoughts and results after the fact:
- Spectral Lines Split: The fine structure makes spectral lines of hydrogen split, like the Balmer series lines.
- Doublets: These are two lines that are very close to each other. They happen because of relativistic effects and spin-orbit interaction.
- Figuring Out How Atoms Are Put Together: The study of fine structure proved that electrons have spin and showed how important relativity is in the very small world.
- Scientists could look at the energy levels of atoms and molecules with a level of clarity that had never been seen before thanks to tools for spectroscopy.
- Fine structure analysis became an important part of spectroscopy.
IMAGE SOURCE (THUMBNAIL)
What's Your Reaction?