Stern-Gerlach Experiment
Learn how the Stern–Gerlach experiment proved quantized angular momentum and electron spin by splitting a silver atom beam in a magnetic field—key to quantum mechanics, superposition, and quantum computing.
Stern-Gerlach Experiment
- A German scientist named Otto Stern and another scientist named Walther Gerlach conducted the Stern-Gerlach experiment in 1922.
- It is one of the most important tests in quantum mechanics because it introduced the idea of quantum states and showed that angular momentum can be quantised.
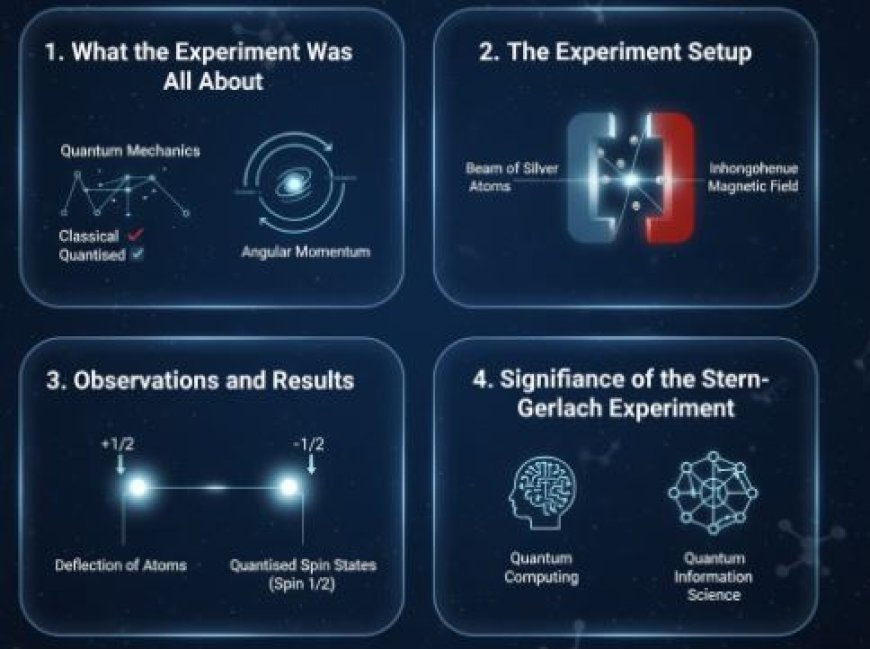
1. What the Experiment Was All About
1.1 About Quantum Mechanics
- What it means: Quantum mechanics is the study of how very small objects, like atoms and subatomic particles, behave.
- Before the Stern-Gerlach experiment, scientists were still trying to figure out how electrons and other particles behaved at the quantum level. This made the experiment essential.
1.2 Angular Momentum
What does angular momentum mean?
- One must consider an object's mass, shape, and speed of spin to determine how much it rotates.
- In classical physics, angular momentum can have any value.
- However, in quantum mechanics, angular momentum is quantised, meaning it can only take on specific values.
2. The Experiment Setup
2.1 Parts That Were Used
- Beam of Silver Atoms: The experiment used a beam of silver atoms.
- These atoms were ideal for studying magnetism because they have only one unpaired electron.
- Inhomogeneous Magnetic Field: The apparatus created a magnetic field that varied in one direction, usually vertically (up and down).
- This uneven field affected each atom's magnetic moment differently.
2.2 Experimental Procedure
Making the Beam:
- Silver metal was heated in a vacuum chamber to produce a beam of moving silver atoms.
- When this beam of silver atoms passed through the inhomogeneous magnetic field, each atom experienced a force due to its magnetic moment.
- Depending on their spin states, the atoms deflected in different directions.
3. Observations and Results
3.1 Deflection of Atoms
- Beam Splitting: Instead of forming a single continuous distribution, the silver atom beam split into distinct spots on a detection screen.
- Quantised Spin States: The deflection pattern showed that the atoms' rotational motion (spin) could only take on specific discrete values.
- It was discovered that silver atoms have a spin of 1/2, which resulted in two distinct spots on the screen.
3.2 Implications of Results
- Introduction of Quantum States: The experiment provided strong evidence for the existence of quantised states in quantum physics.
- Spin Concept: It introduced the idea of "spin", a fundamental property of particles that describes their intrinsic angular momentum.
4. Significance of the Stern-Gerlach Experiment
4.1 Fundamental Discoveries
- Proof for Quantum Theory: The experiment was one of the earliest proofs that particles do not behave classically, confirming the correctness of quantum mechanics.
- Superposition: It contributed to the understanding of superposition, where particles can exist in multiple states simultaneously until measured.
4.2 Applications in Modern Physics
- Quantum Computing:
- Understanding spin helped develop fundamental concepts about quantum states and their manipulation.
- These principles form the basis of quantum computing.
- Quantum Information Science:
- The idea of state splitting is crucial in this field.
- Qubits, which store quantum information, rely on these quantum principles.
IMAGE SOURCE (THUMBNAIL)
What's Your Reaction?