Spin-Orbit Interaction
Spin–orbit interaction describes how an electron’s intrinsic spin couples with its orbital motion around the nucleus. The moving electron generates a magnetic field, which interacts with its own spin, causing energy level splitting known as spin-orbit splitting. This effect helps explain fine structure in atomic spectra, influences electronic properties in materials, and underpins applications such as spintronics and quantum computing.

Spin-Orbit Interaction
Introduction to Spin and Orbit
- In physics, particularly quantum mechanics, we frequently discuss two fundamental features of particles: spin and orbital motion.
- Spin refers to particles' inherent angular momentum.
- Unlike spinning tops or planets, spin has no direct physical counterpart; it is a quantum feature.
- Because of their spin, particles like electrons can be considered small magnets. They can be in one of two states: "up" (+1/2) or "down" (-1/2).
- Orbital Motion: A particle moves around another particle.
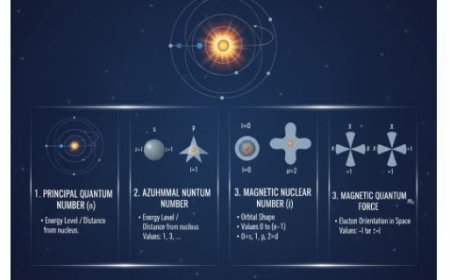
- For example, electrons circle the nucleus of an atom.
- The route and energy of an electron's orbit may be explained using quantum states.
Spin-Orbit Interaction
- Spin-orbit interaction is a fundamental notion in quantum physics that defines the relationship between a particle's spin and orbital motion.
- This indicates that the electron's velocity around the nucleus can influence its spin state, and vice versa.
How Spin-Orbit Interaction Works
1. Magnetic Fields from Motion
- An electron travels around the nucleus, creating a magnetic field.
- This is because a moving charge, like an electron, creates a magnetic field in the same way that a current passing through a wire does.
2. Interaction with Spin
- The electron's velocity produces a magnetic field that interacts with its intrinsic spin.
- This contact can alter the electron's energy levels and even its spin orientation.
3. Energy Splitting
- The interaction splits energy levels into distinct states. This phenomenon is referred to as spin-orbit splitting.
- The degree of splitting is determined by the atomic number of the atom and the unique electron configuration.
The Importance of Spin-Orbit Interaction
Spin-orbit interaction is essential for comprehending numerous physical phenomena and applications.
1. Atomic Structure
- Explains the arrangement of electrons in atoms and how various elements act.
- Affects the fine structure of atomic spectra, critical for understanding light emission and absorption by atoms.
2. Material Science
- Affects the electrical characteristics of materials, especially metals and semiconductors.
- Helps create new materials and technologies, such as improved magnetic materials.
3. Quantum Computing
- Spintronic devices use electron spin instead of charge to build faster and more efficient computer systems.
4. Relativity and High Energy Physics
- High-energy particles can exhibit spin-orbit interaction as a result of relativity.
Key Examples of Spin-Orbit Interaction
1. Rashba Effect
- In systems with significant spin-orbit coupling, an electric field can separate spin states.
2. The Dresselhaus Effect
- Certain materials' crystal lattice symmetry can alter particle spin orientation.
3. Heavy Element
- Heavy elements exhibit stronger spin-orbit interaction due to greater electric fields generated by the nucleus, resulting in major implications on their electronic structure.
IMAGE SOURCE (THUMBNAIL)
What's Your Reaction?