THERMODYNAMIC QUANTITY
Thermodynamic quantities are properties of a system, like temperature, pressure, and volume. They describe the state of the system and how energy flows within it.
Thermodynamic Quantities: Understanding the Language of Heat
- This is an interesting area of physics that looks at how heat, work, temperature, and other interesting aspects of matter and energy are related. It's the study of how things get hot and cold and how those changes can be used to make things that are useful or cool.
- But we need to know how to talk about thermodynamics in order to get around. This is where thermodynamic quantities come in.
- Think of them as the parts in a recipe that will help you understand energy and heat.
- There are different amounts that each describe a different part of how heat energy moves and reacts with matter.
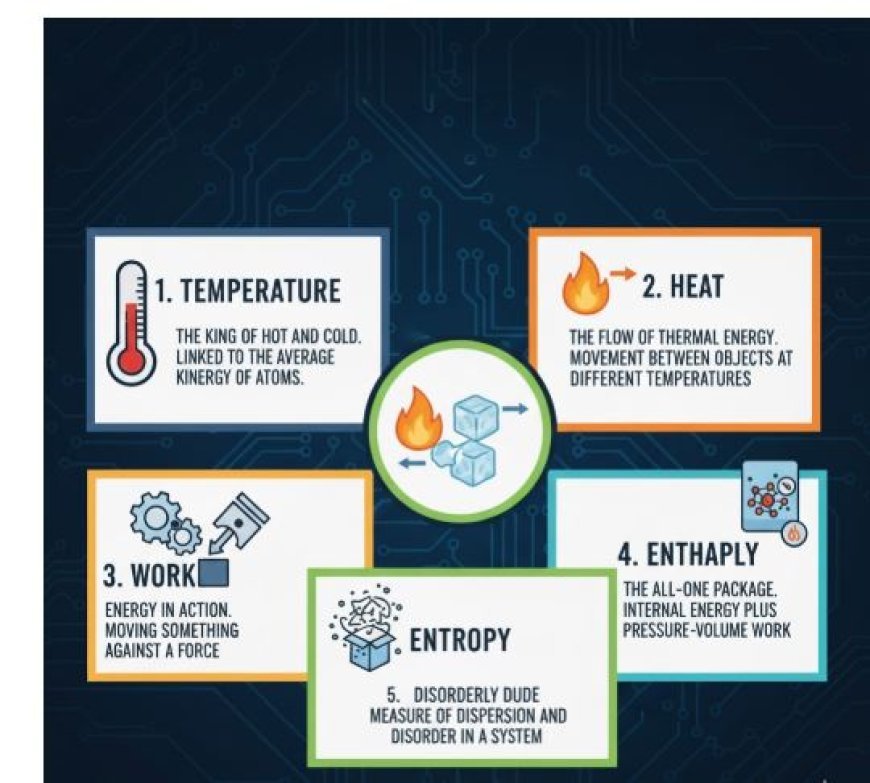
1. Temperature: The King of Hot and Cold
- When it comes to physics, temperature is like the king.
- We can use scales like Celsius and Fahrenheit to find out how hot or cold something is.
- Temperature, on the other hand, is linked to the average kinetic energy of the atoms or molecules that make something up.
- A simple scale measures length.
- The atoms and molecules in hot things move more quickly, while those in cold things move more slowly.
2. Heat: The Flow of Thermal Energy
- When two things at different temperatures move thermal energy from one to the other, this is called heat.
- Like the flow of a river, things are always getting cooler and cooler until they hit balance, which means they are all the same temperature.
- Joules are used to measure heat, and there are three ways it can be sent: directly, through a fluid (convection), or through electromagnetic waves (radiation).
3. Work: Energy in Action
- It takes energy to move something against a force, which is called work.
- Often, the usable energy we get from heat engines or mills is what thermodynamics is all about. The same unit is used to measure work as it is for heat.
- Work can be used for many things, from lifting weights to making power.
4. Enthalpy: The All-in-One Package
- Enthalpy is like a handy group of energies. It is made up of the internal energy of a system (the energy of its atoms and molecules) and the work it can do with pressure and space.
- In chemical processes and phase changes, enthalpy is very important because the total amount of enthalpy stays the same, even though it may be moved or changed.
5. Entropy: The Disorderly Dude
- Entropy is like a naughty monster that likes to make things more chaotic. It shows how dispersed and disorganized the energy is in a system.
- The second law of thermodynamics says that entropy tends to rise over time. This means that higher entropy means more chaos. This is the reason why it's easy to make your room messy but tough to keep it spotless.
What's Your Reaction?