The Vector Model of the Atom and Its Quantum Numbers
Learn how the vector model explains atomic structure through quantized angular momentum and four quantum numbers that define electron identity, orbital shape, and spin.
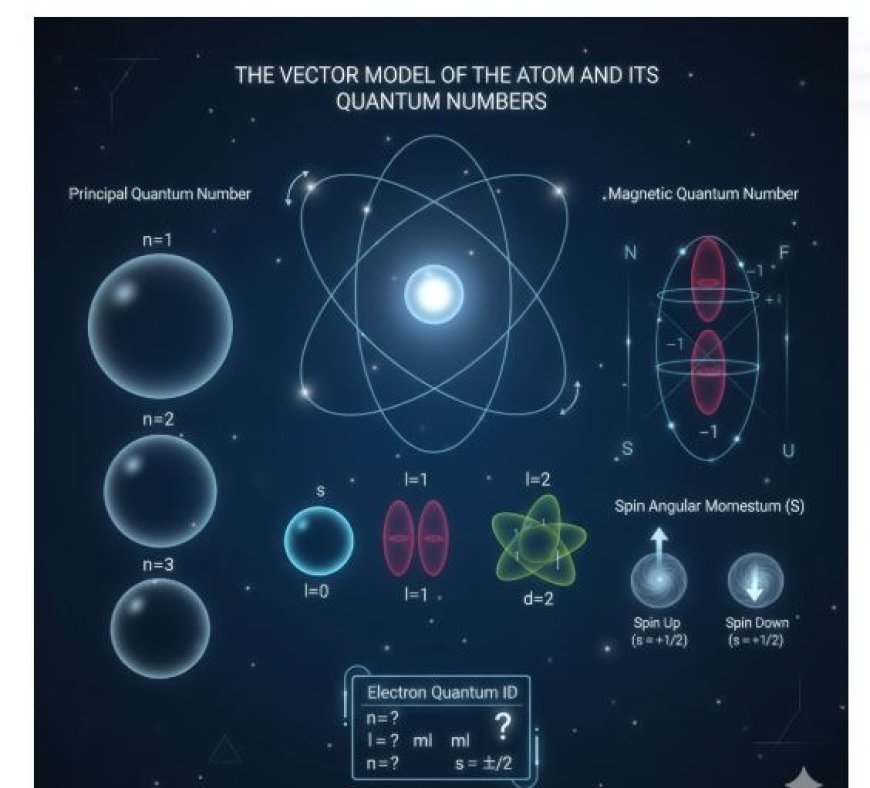
The Vector Model of the Atom and Its Quantum Numbers
- For hundreds of years, scientists have been interested in the atom, which is the building block of all matter.
- Modern physics has shown us a much more complicated and interesting picture of the atom than the simple spheres that were used in the past.
- The vector atom model tells us everything we need to know about how atoms work by focusing on the magnetic and rotational momentum properties of electrons.
Angular Momentum in the Vector Atom Model
- The vector atom model is based on the idea of rotating motion.
- Think of an electron as a small planet orbiting around the centre.
- This orbital motion has a built-in rotating force, like a spinning top.
- However, quantum mechanics controls how electrons behave inside an atom, making them unique:
-
- Quantisation of Angular Momentum: Quantum physics states that angular momentum is quantised, meaning it exists in discrete amounts rather than a continuous range.
- In other words, an electron’s rotational momentum can only have specific values.
- Orbital Angular Momentum (L): This refers to the rotational momentum of an electron moving in a circular orbit around the nucleus. It is represented by the azimuthal quantum number (l).
- It can have values ranging from 0 to (n-1), where n is the principal quantum number.
- In chemistry, different values of l correspond to different atomic orbitals:
- l = 0 → s orbital (spherical shape)
- l = 1 → p orbital (dumbbell shape)
- l = 2 → d orbital, and so on.
- Spin Angular Momentum (S):
- Electrons do not spin like a physical object, but they have an intrinsic spin that contributes to their angular momentum.
- This spin angular momentum is represented by the spin quantum number (s).
- For electrons, s is always 1/2.
Quantum Numbers and Electron Identification
The vector atom model uses four quantum numbers to describe an electron’s behavior inside an atom. Each electron has a unique set of quantum numbers, like an ID card, which provides information about its energy, orbital shape, and spatial orientation.
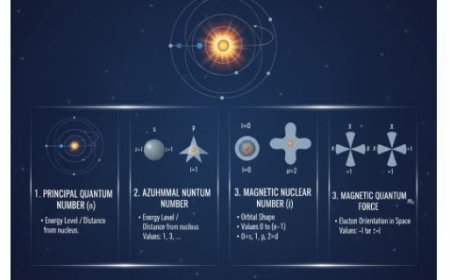
1. Principal Quantum Number (n):
- Represents an electron’s energy level or shell.
- It can take any positive integer value (1, 2, 3, etc.).
- Larger 'n' values correspond to higher energy levels and larger electron orbitals.
2. Azimuthal (Orbital) Quantum Number (l):
- Defines the shape of the orbital.
- It can take values from 0 to (n-1).
- Different l values correspond to different orbital shapes:
- l = 0 → s orbital (spherical)
- l = 1 → p orbital (dumbbell-shaped)
- l = 2 → d orbital, etc.
3. Magnetic Quantum Number (ml):
- Determines the orientation of the orbital in space relative to an external magnetic field.
- It can take integer values ranging from -l to +l, including 0.
- Example:
- If l = 1 (p orbital), then ml can be -1, 0, or +1, meaning the p orbital can have three different spatial orientations.
IMAGE SOURCE(THUMBNAIL)
What's Your Reaction?