Microwave and Infrared (IR) Spectroscopy: Molecular Classification
Microwave and infrared (IR) spectroscopy are powerful techniques used to study how molecules interact with electromagnetic radiation. Microwave spectroscopy focuses on rotational transitions to classify molecules—especially diatomic types—while IR spectroscopy identifies vibrational modes and functional groups such as –OH and C=O. These methods help determine chemical structure, detect gases, and support research and industrial quality control.
Microwave and Infrared (IR) Spectroscopy: Molecular Classification
Introduction
Spectroscopy
- Spectroscopy is the study of light's interactions with materials. When light interacts with a molecule, it can be absorbed, emitted, or dispersed.
- Scientists can learn a lot about the structure and behaviour of molecules by studying their interactions.
Types of Spectroscopy
- Microwave spectroscopy
- Infrared spectroscopy
Microwave Spectroscopy
How It Works
- Microwave spectroscopy investigates the absorption of microwave radiation by molecules.
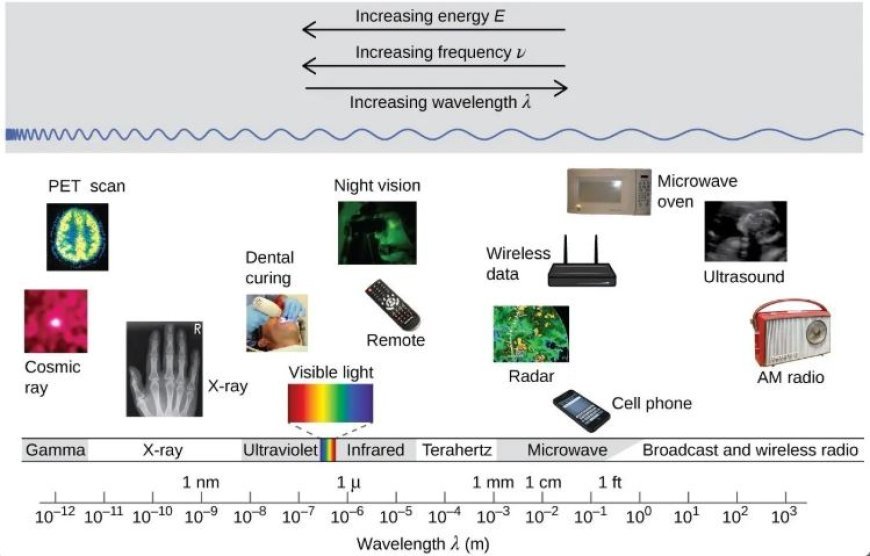
- Microwaves are a kind of electromagnetic radiation that has longer wavelengths than visible light.
Key Points
- Energy Levels: Each molecule has a particular energy level. Microwaves may force the bonds of a molecule to rotate, transferring energy.
- Rotational Transitions: The energy absorbed corresponds to the difference in rotational energy levels inside a molecule.
Molecule Classification
- Microwave spectroscopy is very effective for categorising diatomic compounds (molecules with two atoms). This is how we may categorise them.
- Homodiatomic Molecules: Examples are O₂ (Oxygen) and N₂ (Nitrogen). These molecules contain identical atom kinds.
- Heterodiatomic Molecules: Examples are CO (Carbon Monoxide) and HCl (Hydrochloric Acid). These molecules include two kinds of atoms.
Applications
- Identify Gases: Used in atmospheric investigations to determine gas composition.
- Research: Aids in understanding reactions and dynamics in physical chemistry.
Infrared (IR) Spectroscopy
- Infrared spectroscopy uses infrared light to stimulate the vibrations of atoms in a molecule. IR radiation's wavelengths are longer than visible light but shorter than microwaves.
Key Points
- Molecules may vibrate in many ways, including stretching and bending.
- When infrared light strikes a molecule, specific wavelengths are absorbed, resulting in these vibrations.
- Functional Groups: Scientists may identify bonds and chemical structures based on their IR wavelength absorption.
Molecule Classification
- IR spectroscopy is extremely useful for categorising a large variety of chemicals, particularly organic compounds. Here's how you can categorise molecules:
- Homogeneous Molecules:
- Have the same type of atoms.
- Examples: CH₄ (methane) and C₂H₆ (ethane).
- Heterogeneous Molecules:
- Made up of many sorts of atoms.
- Example: Ethylene Oxide (C₂H₄O).
- Functional Groups:
- Various functional groups exhibit distinct IR absorption patterns.
- Examples of high absorption are the hydroxyl group (-OH) around 3200-3600 cm⁻¹ and the carbonyl group (C=O) about 1700 cm⁻¹.
Applications
- Identifying Unknown Chemicals: A common laboratory practice.
- Quality Control: A process used in companies to ensure product purity.
What's Your Reaction?