Zeeman Effect and Paschen Back Effect
The Zeeman and Paschen-Back effects describe how magnetic fields split atomic spectral lines. In weak fields, the Zeeman effect produces simple line splitting, while strong fields cause the more complex Paschen-Back effect. In two-electron systems, spin states, orbital motion, and selection rules determine splitting patterns, helping analyze atomic structure and stellar magnetic fields.
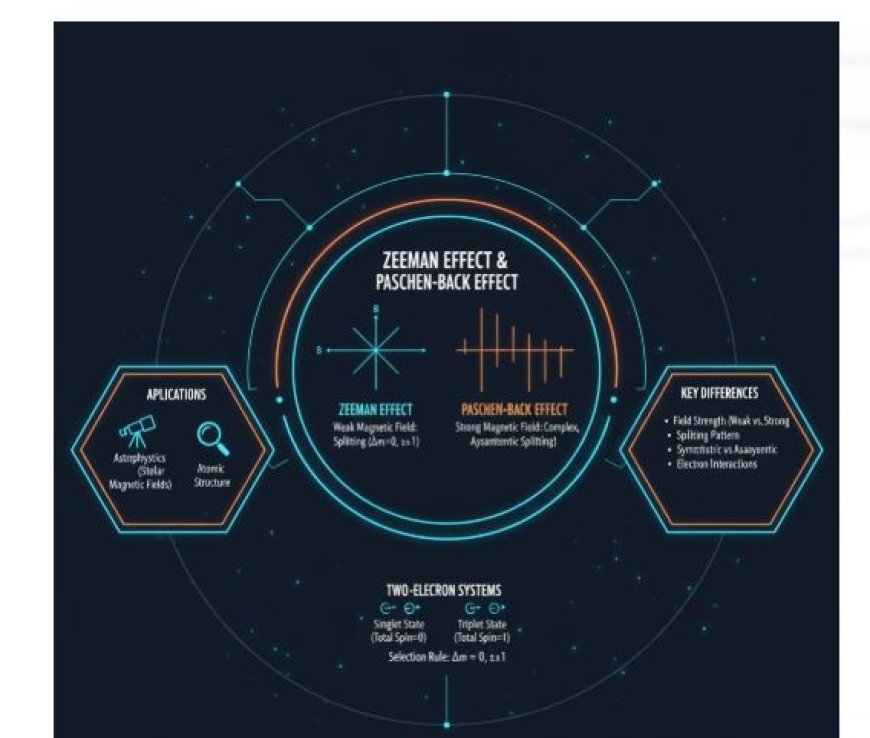
The Zeeman Effect and the Paschen-Back Effect in Systems with Two Electrons
Figuring out how electrons move in a magnetic field can lead to interesting discoveries in physics. The Zeeman effect and the Paschen-Back effect are two major effects that demonstrate this phenomenon.
1. What is the Zeeman Effect?
- Different spectral lines are split when a magnetic field interacts with the magnetic moment of atoms or molecules. This is called the Zeeman effect.
1.1 Important Things to Know About the Zeeman Effect
What it is:
- An electron's "spin", which creates a magnetic moment, is responsible for the effect.
- When a magnetic field is present, the energy levels of electrons change. This results in the splitting of emitted or absorbed light into several closely spaced lines.
1.2 Normal Zeeman Effect
- This occurs when electrons are at the same energy level.
- There are three parts to the splitting:
- One in the middle
- Two on either side
- The strength of the magnetic field affects the extent of the splitting.
1.3 Conditions
- Weak Magnetic Field: The normal Zeeman effect is more noticeable when the magnetic field is weak.
- Atomic Transitions: The wavelengths of light that are emitted or absorbed are determined by energy differences during electronic transitions.
1.4 Uses of the Zeeman Effect
- Astrophysics: Observing spectral lines helps in understanding the magnetic fields of stars.
- Understanding Atomic Structure: It allows scientists to study the internal structure of atoms and molecules.
2. What is the Paschen-Back Effect?
- The Paschen-Back effect is an extension of the Zeeman effect that occurs in the presence of stronger magnetic fields.
2.1 Important Things to Know About the Paschen-Back Effect
- Stronger Fields: This occurs when the magnetic field is so strong that it overpowers the interaction between electron spin and orbital motion.
- Changes in Energy Levels: The Paschen-Back effect causes more complex splitting patterns than the Zeeman effect.
2.2 What Makes the Paschen-Back Effect Different from the Zeeman Effect?
- The splitting pattern in the Paschen-Back effect is not symmetric.
- It is more complex due to additional interactions between quantum states.
3. Systems with Two Electrons
- In systems with two electrons, both the Zeeman effect and the Paschen-Back effect can be observed.
- These systems are more complex due to the interactions between spin and orbital motion.
3.1 Electron Spins
- When two electrons are paired, their total spin is zero.
- When they are unpaired, their total spin is not zero.
- Spin states:
- Singlet state: The total spin = 0.
- Triplet state: The total spin = 1.
- These spin states influence the selection rules for transitions.
3.2 Atomic Configurations
- The way a magnetic field interacts with two electrons depends on their arrangement:
- Same orbital or different orbitals
- Determines the spectral lines produced
4. Rules for Choosing Transitions
- Quantum number changes determine whether transitions between energy states are allowed or forbidden.
4.1 Selection Rules
- Spin Change:
- For magnetic transitions, changes in total angular momentum (J) must follow specific rules.
- Zeeman Effect:
- Transitions are usually allowed when Δm = 0, ±1.
- Paschen-Back Effect:
- In strong fields, larger changes may occur depending on the system's energy states.
4.2 Implications
- Understanding selection rules helps predict which transitions will occur in experiments.
- Helps scientists interpret spectra and analyze atomic and molecular structures.
What's Your Reaction?



































