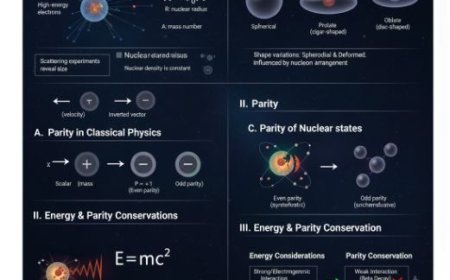
Nuclear Reactions and Models
Understand how nuclear reactions release or absorb energy through fission, fusion, and decay. Learn about Q-value, threshold energy, and key nuclear models like the Liquid Drop Model, Shell Model, and Collective Model that explain nuclear stability and behavior.
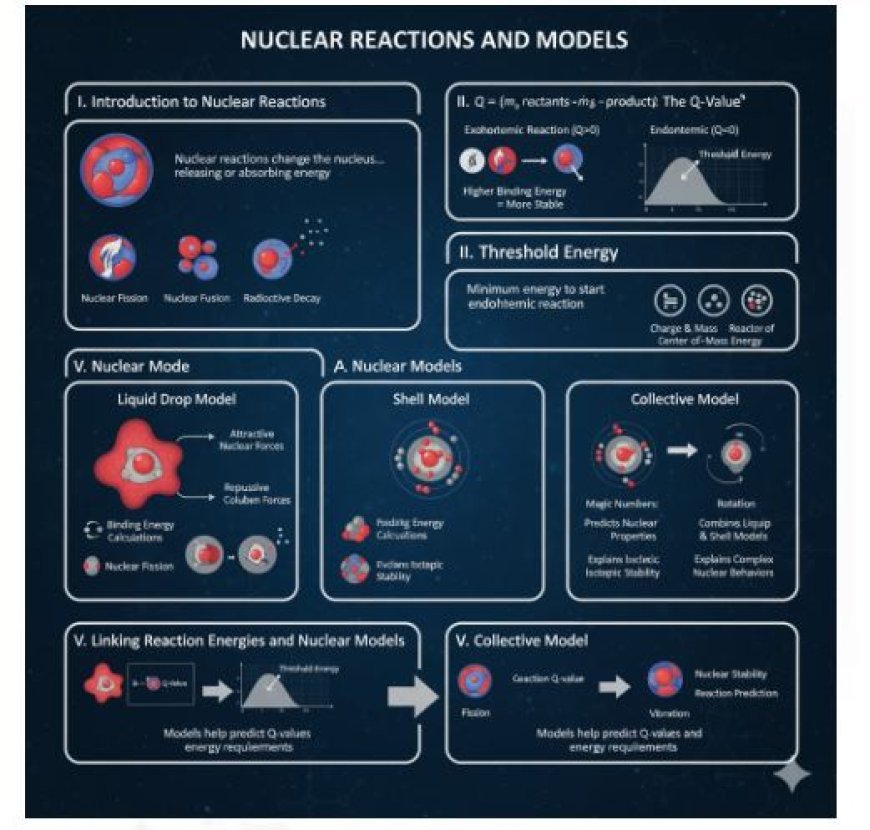
Nuclear Reactions and Models
I. Introduction to Nuclear Reactions
Atoms consist of a central nucleus, which contains protons and neutrons, and is surrounded by electrons. Nuclear reactions change the nucleus by adding or removing protons and/or neutrons. This differs from chemical reactions, which involve only electrons. Nuclear changes can release or absorb large amounts of energy.
Examples of nuclear reactions include:
- Nuclear fission: Breaking apart a heavy atomic nucleus.
- Nuclear fusion: Joining together light atomic nuclei.
- Radioactive decay: The nucleus emits particles on its own.
II. Energy in Reactions: The Q-Value
The Q-value shows how much energy is released or absorbed in a nuclear process:
- A positive Q-value means energy is released (exothermic reaction).
- A negative Q-value means energy is absorbed (endothermic reaction).
• Calculating the Q-Value
The Q-value is found by calculating the difference between the total mass of reactants and the total mass of products. Einstein’s famous equation, E = mc², shows that this mass difference corresponds to energy release. When mass decreases, energy is released.
• Mass Defect and Binding Energy
- The mass defect is the difference between the actual mass of a nucleus and the total mass of its nucleons (protons + neutrons).
- This missing mass is converted into binding energy, which holds the nucleus together.
- A higher binding energy per nucleon means the nucleus is more stable.
III. Threshold Energy
· For some endothermic nuclear reactions (which absorb energy), a minimum amount of energy must be supplied to start the reaction. This is called the threshold energy. It is needed to overcome repulsive forces between reactants (especially in reactions with positively charged particles).
• Factors Influencing Threshold Energy
Threshold energy depends on:
- Charge and mass of the reacting particles.
- Reaction Q-value (a more negative Q-value requires higher energy).
- Center-of-mass energy, which is the energy available for the reaction when viewed from a non-moving reference frame.
IV. Nuclear Models
• Why Do We Use Models?
Nuclear behavior is complex, so models help simplify explanations and predict outcomes.
• Liquid Drop Model
- This model treats the nucleus like an incompressible liquid drop.
- It explains nuclear binding energy and helps describe fission processes.
• Shell Model
- Suggests that protons and neutrons (nucleons) are arranged in specific energy levels, similar to how electrons are organized in an atom.
- Nuclear stability depends on certain proton and neutron numbers called magic numbers (e.g., 2, 8, 20, 28, 50, 82, 126).
- Nuclei with magic numbers are more stable and less likely to decay.
• Collective Model
- This model combines ideas from the Liquid Drop Model and Shell Model.
- It explains how atomic nuclei move together, such as spinning and vibrating.
V. Linking Reaction Energies and Nuclear Models
- Nuclear models help predict Q-values and minimum energy requirements for nuclear reactions.
- The Liquid Drop Model explains binding energy and predicts energy release during fission.
- The Shell Model helps understand nuclear stability and predicts whether certain nuclear reactions will occur.
What's Your Reaction?