ZEEMAN EFFECT
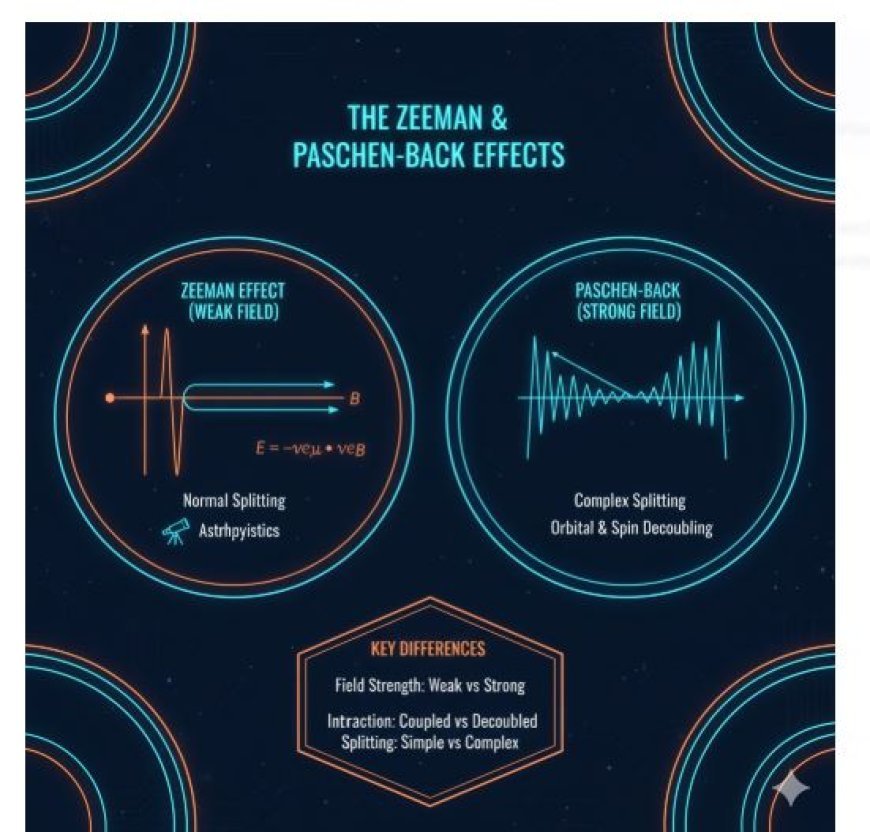
The Zeeman Effect describes how weak magnetic fields split atomic spectral lines into multiple components due to interactions with electron magnetic moments. In contrast, the Paschen-Back Effect occurs under strong magnetic fields, causing more complex splitting as orbital and spin angular momenta decouple. Together, these effects reveal how magnetic fields reshape atomic energy levels, advancing spectroscopy, quantum mechanics, and astrophysics.
The Zeeman Effect
1. Historical Background
- When there is a strong magnetic field around, spectral lines split apart. This is called the Zeeman effect.
- Background: Pieter Zeeman found this effect in 1896 and won the Nobel Prize in Physics for it in 1902.
- It helped us learn more about spectroscopy and showed how magnetic fields can change the way atoms behave.
2. The Basic Idea
- When there isn't a magnetic field around, atoms give off light at certain wavelengths that show changes in energy levels.
- When a magnetic field is applied, these energy levels move because the magnetic field interacts with the electrons' magnetic moments.
3. An Explanation of the Result
- Energy Level Splitting: Atoms' energy levels split into several smaller levels, called Zeeman sub-levels. This makes a spectrum with many lines that are very close to each other.
The Normal and Anomalous Zeeman Effect:
- The normal Zeeman effect is seen in light as a single spectral line that splits into three parts (the π and σ lines) when a weak magnetic field is introduced.
- Anomalous Zeeman Effect: This happens when fine structure is present; it can lead to more than three components and is caused by extra problems in the structure of the energy levels.
4. Treatment with Quantum Mechanics
- The Hamiltonian operator lets us describe how the magnetic moments and the magnetic field interact using quantum mechanics.
- The energy of a magnetic moment in a magnetic field (B) is given by E_B = -vecμ ⋅ vecB, where (vecμ) is the magnetic moment vector.
- The changes in energy cause different spectral lines to show up, which can be analysed using the rules of quantum physics.
The Paschen-Back Effect
- People have seen the Paschen-Back effect when the magnetic field strength is a lot bigger than when the Zeeman effect is happening.
1. History
- It was named for Friedrich Paschen and Hermann Back, two scientists who did a lot of research on it in the early 1900s.
- In this case, the magnetic field is strong enough to separate the orbital and spin magnetic moments of electrons fully.
2. Primary Features
- The Zeeman effect has pretty simple line splitting, but the Paschen-Back effect has more complicated patterns of line splitting.
- When strong magnetic fields are applied, the spectral lines will change their order, which means that the electrons will be in a different quantum state.
3. Mechanism of the Effect
- A strong magnetic field changes the way that the magnetic moments of electrons interact with each other.
- The electrons are mostly affected by the nucleus's magnetic field, not its electric field. This changes the way their energy is organised.
4. Quantum Mechanical Treatment
- The Hamiltonian for the Paschen-Back effect needs a more complicated way of dealing with the electron states, taking into account both the spin and the angular momentum of the orbital.
- The transitions between the new sub-levels will be shown by the results of quantum physics.
Zeeman and Paschen-Back Effects Side by Side
1. Field Strength
- The Zeeman Effect can be seen when magnetic fields are weak.
- The Paschen-Back Effect needs strong magnetic fields to work.
2. Interaction of Energy Levels
- The Zeeman Effect involves the mixing of orbital and spin states.
- The Paschen-Back Effect is dominated by magnetic field interactions, making states stand out more.
3. Patterns for Splitting Lines
- Zeeman Effect: Simple splitting into more than one line.
- Paschen-Back Effect: Arrangements that are more complicated with more lines.
What's Your Reaction?