Types of Nuclear Reactions
Discover the major types of nuclear reactions, including fission, fusion, radioactive decay, capture reactions, and spallation. Learn how they work and their applications in energy, medicine, and astrophysics.
Types of Nuclear Reactions
Nuclear reactions are interesting processes that occur at the level of atoms and their individual particles. Understanding these processes helps us learn about different things in physics, science, and our daily lives.
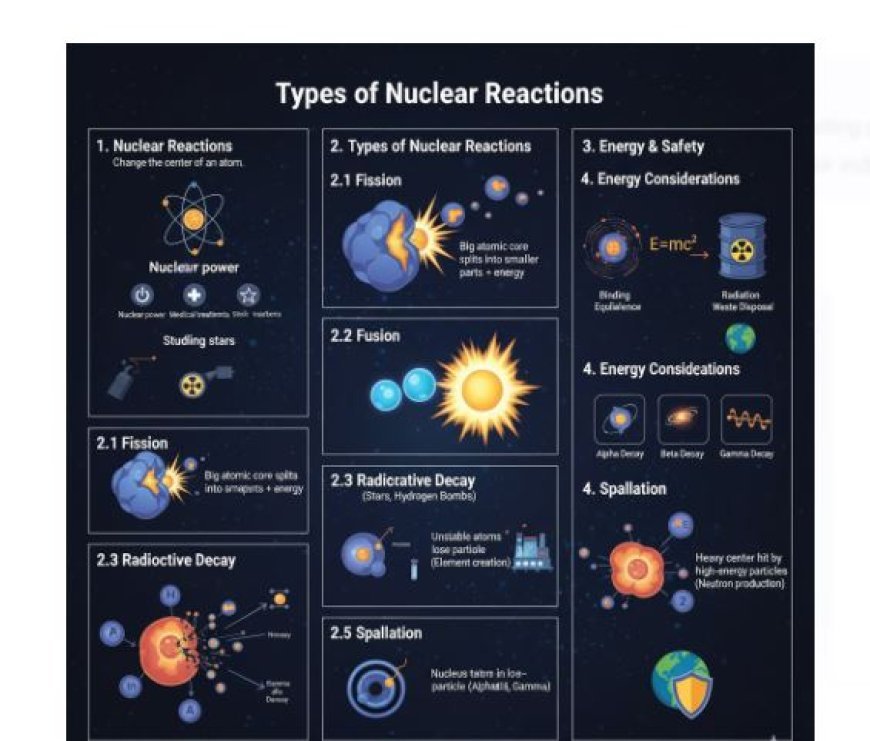
1. Nuclear Reactions
Definition: Nuclear processes change the center of an atom, turning one element into another or creating new particles.
Nuclear reactions are important for things like:
- Generating nuclear power
- Medical treatments (like radiation)
- Studying stars
2. Types of Nuclear Reactions
Nuclear events can be grouped into different types based on the processes and particles involved.
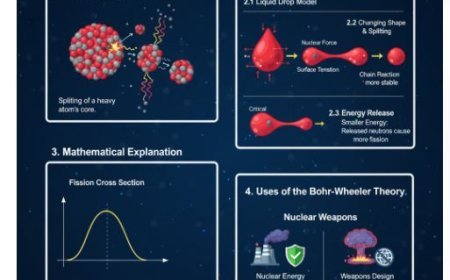
2.1 Fission
Fission is when a big atomic core splits into smaller parts, and this produces energy.
- Process: The process uses heavy elements like uranium-235 or plutonium-239. When these elements are hit with neutrons, they become unstable, split apart, and release more neutrons and energy.
- Uses:
- Producing energy in nuclear plants
- Atomic bombs
2.2 Fusion
- Definition: Fusion is when two light atomic nuclei join together to create a heavy nucleus and release energy in the process.
- Process: Happens at very high temperatures and pressures, like in the center of stars (for example, when hydrogen combines to form helium).
- Uses:
- Can provide clean energy from controlled fusion processes
- Involved in hydrogen bombs
2.3 Radioactive Decay
- Definition: A natural process where unstable atoms lose energy by releasing radiation in the form of particles or electromagnetic waves.
Types of Decay:
- Alpha Decay: Emission of alpha particles (two protons and two neutrons).
- Beta Decay: This is when a neutron changes into a proton (beta-minus) or a proton changes into a neutron (beta-plus), releasing beta particles in the process.
- Gamma Decay: The release of gamma rays (high-energy light) from a nucleus that is excited, without changing the number of particles in it.
2.4 Nuclear Capture Reactions
- Definition: Reactions where a nucleus takes in a particle, resulting in a larger nucleus.
- Example: Neutron capture happens when a nucleus takes in a neutron, making a heavier version of that element.
- Applications:
- Crucial for making elements in stars
- Used for creating certain medical isotopes
2.5 Spallation
- Definition: This is a nuclear process where a heavy center is hit by high-energy particles, which makes it release smaller particles like protons, neutrons, or alpha particles.
- Applications:
- Used in particle reactors to create neutrons
- Applied in some nuclear studies
3. Energy Considerations in Nuclear Reactions
- Binding Energy: The energy that keeps the center intact. A change in binding energy during a process leads to either the release or absorption of energy.
- Mass-Energy Equivalence: Explained by Einstein's equation (E=mc²), which states that mass can change into energy during nuclear processes.
4. Safety and Environmental Issues
- Radiation: Nuclear processes can create dangerous radiation, so safety precautions are needed in nuclear power plants and medical uses.
- Waste Disposal: Proper handling of nuclear waste is important to reduce harm to the earth.
IMAGE SOURCE (THUMBNAIL)
What's Your Reaction?