HUNDS RULE
Hund’s Rule explains how electrons fill degenerate orbitals—occupying them singly with parallel spins before pairing—to maximize stability. This principle shapes atomic structure, chemical behavior, magnetism, and reactivity, and supports understanding of energy levels, spectra, and quantisation in quantum physics and astrophysics.

Hund's Rule
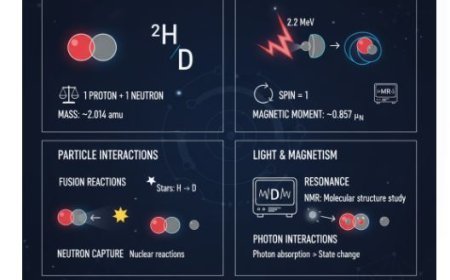
• Astrophysics: Alkali spectra are used to evaluate the composition of stars and other celestial bodies by measuring the light they produce or absorb.
• Understanding alkali spectra can help you learn about atomic transitions, energy levels, and fundamental concepts such as quantisation.
• Understand Hund's Rule of Physics. Hund's Rule serves as the foundation for quantum physics and atomic theory, describing how electrons in atoms are arranged.
• The arrangement of these atoms is important because it affects how molecules form and the chemical properties of elements.
• Hund’s Rule: According to Hund's Rule, degenerate orbitals have the same energy level, therefore electrons will occupy them individually before pairing up.
• When two orbitals have the same amount of energy, electrons prefer to remain alone and spin in the same direction before partnering up.
- Astrophysics: Alkali spectra are used to evaluate the composition of stars and other celestial bodies by measuring the light they produce or absorb. Understanding alkali spectra can help you learn about atomic transitions, energy levels, and fundamental concepts such as quantisation.
- Understand Hund's Rule of Physics. Hund's Rule serves as the foundation for quantum physics and atomic theory, describing how electrons in atoms are arranged. The arrangement of these atoms is important because it affects how molecules form and the chemical properties of elements.
- Hund’s Rule: According to Hund's Rule, degenerate orbitals have the same energy level, therefore electrons will occupy them individually before pairing up. When two orbitals have the same amount of energy, electrons prefer to remain alone and spin in the same direction before partnering up.
Important Aspects of Hund's Rule
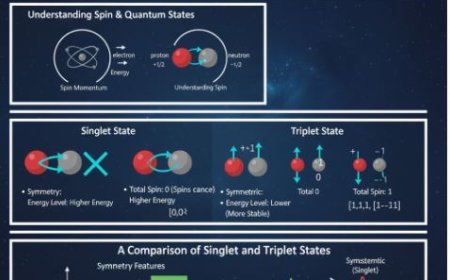
1. Orbitals That Break Down
- These are orbitals with the same amount of energy.
- In a p subshell, all three p orbitals (px, py, and pz) are degenerate and have the same energy.
- Similarly, the five d orbitals in a d subshell are degenerate.
- When the degenerate orbitals are filled, they get one electron first.
- We do not start coupling electrons (adding a second electron to each orbital) until all orbitals have one electron.
- Spin is a property of electrons that may be characterised as a small magnetic moment.
- According to Hund's Rule, the first electron in each degenerate orbital will have the same spin (also known as "up" spin) before any pairs occur.
What Is the Significance of Hund's Rule?
Hund's Rule is critical for understanding how electrons are organised.
1. Stability
- Having electrons in different orbitals reduces the force that pushes them apart, making electron grouping more stable.
- This stability is crucial in understanding how chemicals interact with atoms.
2. Predicting Chemical Behaviour
- Understanding how electrons are structured enables scientists to anticipate how elements will bond together.
- Unpaired electrons, for example, make metals more reactive.
3. Magnetism
- The Hund's Rule-based arrangement of electrons may also indicate whether a material is paramagnetic (attractive to magnets owing to unpaired electrons) or diamagnetic (unattractive to magnets because all electrons are paired).
Examples of Hund's Rule in Action
Consider how electrons are distributed in different elements to see how Hund's Rule works.
1. Oxygen (O₂) (Atomic Number 8)
- The electron configuration of oxygen is 1s² 2s² 2p⁴.
- The 2p subshell consists of three degenerate p orbitals.
- Hund's Rule states that one electron will fill each of the three orbitals before pairing commences.
- This links two electrons in a p orbital.
2. Nitrogen (N) (Atomic Number 7)
- The 2p subshell of nitrogen has three electrons that all face the same direction ("up").
- This form makes nitrogen fairly stable, which is why it is included at that place on the periodic table.
Important Aspects of Hund's Rule
1. Orbitals that Break Down
- These are orbitals with the same amount of energy.
- In a p subshell, all three p orbitals (px, py, and pz) are degenerate and have the same energy.
- Similarly, the five d orbitals in a d subshell are not symmetrical.
- When the degenerate orbitals are filled, they get one electron first.
- We do not start coupling electrons (adding a second electron to each orbital) until all orbitals have one electron.
- Spin is a property of electrons that may be characterised as a small magnetic moment
- According to Hund's Rule, the first electron in each degenerate orbital will have the same
spin (also known as "up" spin) before any pairs occur.
What is the Significance of Hund's Rule?
Hund's Rule is critical for understanding how electrons are organised.
1. Stability
- Having electrons in different orbitals reduces the force that pushes them apart, making electron grouping more stable.
- This stability is crucial in understanding how chemicals interact with atoms.
2. Predicting Chemical Behaviour
- Understanding how electrons are structured enables scientists to anticipate how elements will bond together.
- Unpaired electrons, for example, make metals more reactive.
3. Magnetism
• The Hund's Rule-based arrangement of electrons may also indicate whether a material is paramagnetic (attractive to magnets owing to unpaired electrons) or diamagnetic (unattractive to magnets because all electrons are linked).
Examples of Hund's Rule in Action
Consider how electrons are distributed in different elements to see how Hund's Rule works.
- Oxygen (O₂) (Atomic Number 8): The electron configuration of oxygen is 1s² 2s² 2p⁴. The 2p subshell consists of three degenerate p orbitals. Hund's Rule states that one electron will fill each of the three orbitals before pairing commences. This links two electrons in a p orbital.
- Nitrogen (N) (Atomic Number 7): The 2p subshell of nitrogen has three electrons that all face the same direction ("up"). This form makes nitrogen fairly stable, which is why it is included at that place on the periodic table.
What's Your Reaction?