Born-Oppenheimer Approximation
The Born–Oppenheimer Approximation simplifies molecular quantum mechanics by separating fast-moving electrons from slow-moving nuclei based on their mass difference. This reduces complex many-particle interactions, enabling easier calculations of electronic states, potential energy surfaces, and molecular motion. Widely used in chemistry, spectroscopy, and material science, though less accurate when electron–nuclear coupling is strong.
Born-Oppenheimer Approximation
- An important idea in quantum mechanics, especially in molecular physics and chemistry, is the Born-Oppenheimer estimate.
- When you separate the movements of nuclei and electrons, it makes molecular processes easier to understand.
- The ideas behind the Born-Oppenheimer approximation will be broken down in a clear and simple way in this piece.
1. Introduction to the Born-Oppenheimer Approximation
- What it means: In quantum chemistry, the Born-Oppenheimer approximation is a way to make the complicated interactions between systems with many particles, like molecules, easier to understand by assuming that the motions of nuclei and electrons can be handled independently.
- History: This idea was first put forward by scientists Max Born and Robert Oppenheimer in the early 1900s, which helped modern quantum chemistry grow.
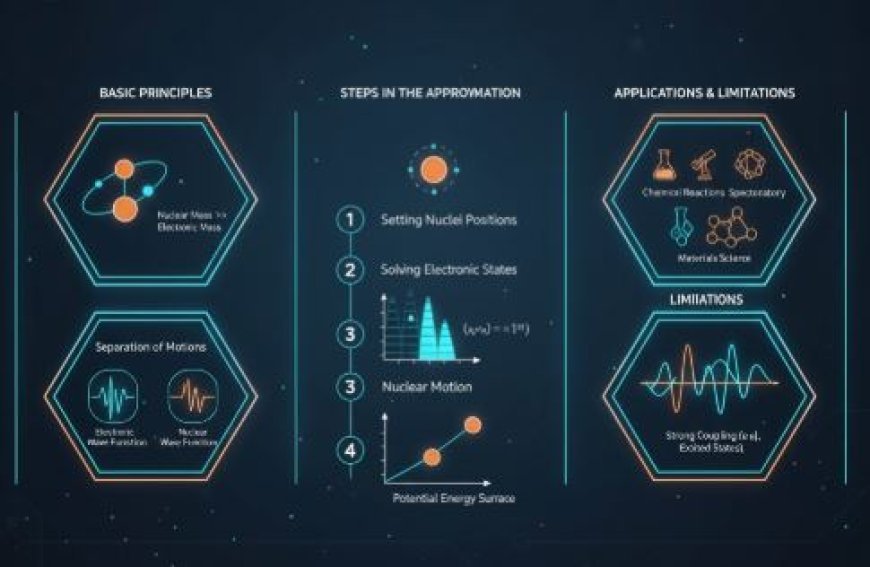
2. Basic Principles
- Nuclear Mass vs. Electronic Mass:
- Nuclei are much heavier than electrons in molecules.
- Because of this big difference in mass, nuclei move much more slowly than electrons.
- Separation of Motions:
- The approximation takes into account this difference in mass, which lets scientists separate the motion of nuclei and electrons.
- The total wave function of a molecular system can be written as the result of:
- An electronic wave function that is based on electronic coordinates.
- A nuclear wave function that is based on nuclear coordinates.
3. Steps in the Born-Oppenheimer Approximation
Step 1: Setting the Positions of the Nuclei
- The positions of the nuclei are kept fixed when figuring out how the electrons in a molecule behave.
- This makes it possible to simplify the Schrödinger equation and only look at the electrons.
Step 2: Solving for Electronic States
- If you set the nuclei, you can solve the electronic wave functions.
- These wave functions give you the electronic energies as a function of where the nuclei are fixed.
Step 3: Nuclear Motion
- The nuclei can move after the electric states have been found.
- The energies from the electronic solutions are used to make potential energy surfaces for the atoms.
- This lets you figure out how the nucleus is moving using either classical or quantum physics.
4. The Born-Oppenheimer Approximation Has Some Benefits
- Makes calculations easier:
- Complex many-body problems can be solved by separating the movements of nuclei and electrons.
- Use in Molecular Dynamics:
- Potential energy surfaces obtained from electronic states can be used to study nuclear motion.
- This makes it easier for researchers to study how molecular systems change over time.
5. Limitations
- Here's a breakdown of close interactions:
- The approximation might not work when the movements of electrons and nuclei are strongly connected.
- This happens in cases such as excited states changes or when nuclei are very light.
- Not Good Enough for Some Systems:
- The estimate can be wrong for some molecules, especially those where the nuclei and electrons are strongly coupled in vibrational or rotational ways.
6. Applications
- Understanding transition states and reaction paths in chemical reactions is an important part of learning about reactions.
- Spectroscopy: Reading energy levels and changes in molecular spectra to learn more about them.
- Material science: The study of the qualities of complicated materials and the creation of new compounds.
What's Your Reaction?