BONDING AND ANTIBONDING MOS
Discover how molecular orbital theory explains bonding and antibonding orbitals. Bonding orbitals result from constructive overlap of atomic orbitals, increasing electron density between nuclei and stabilizing molecules. Antibonding orbitals arise from destructive overlap, decreasing electron density and weakening bonds. Understanding these orbitals helps predict molecular stability, electron configuration, and reactivity.

Molecular Orbitals That Bond and Break Bonds
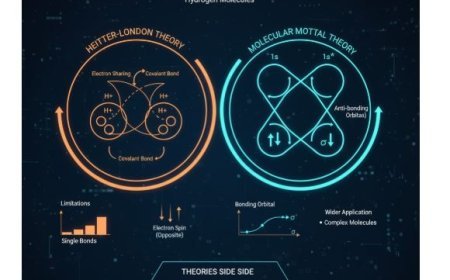
Molecular orbital theory is one of the most fundamental concepts in chemistry and physics. It depicts how atoms unite to form molecules. This hypothesis distinguishes two sorts of molecular orbitals: those that bond and those that do not bond.
1. Molecular Orbitals
- Molecular orbitals (MOs) are regions in a molecule where electrons are expected to exist. When atoms combine to form a molecule, their atomic orbitals mix.
- There are two primary categories of MOs:
- Bonding molecular orbitals
- Antibonding molecular orbitals
2. Molecular Orbitals That Bond
- What this means:
-
- Bonding molecular orbitals are formed when atomic orbitals interact in a way that strengthens them.
- In other words, there are more electrons in the space between two atoms.
2.1 Properties of Bonding Orbitals
- Bonding orbitals are lower in energy than the atomic orbitals they are formed from.
- Atoms bind together for a variety of reasons, including stability.
- Higher electron density:
- The majority of electrons in a bonding orbital are located between two atomic nuclei.
- This draws the nuclei closer together, resulting in a stronger bond.
- Bonding orbitals are often written with a lowercase sigma (σ) or pi (π).
2.2 Types of Bonding Orbitals
- Sigma (σ) orbitals:
- Formed when atomic orbitals collide head-on.
- Found in single bonds and have a cylindrical shape around the bond axis.
- Pi (π) orbitals:
- Found in double and triple bonds, along with sigma bonds.
- Formed when p-orbitals overlap laterally.
3. Antibonding Molecular Orbitals
- What this means:
- Antibonding molecular orbitals are formed when atomic orbitals overlap destructively, leading to cancellation of electron density.
- This results in a reduction of electron density between two atoms, making the bond weaker.
3.1 Characteristics of Antibonding Orbitals
- Higher Energy:
- Antibonding orbitals have higher energy than the original atomic orbitals.
- This increased energy makes molecules less stable.
- Lower Electron Density:
- When electrons occupy an antibonding orbital, they create a node (a region of zero electron density) between the nuclei.
- This pushes the nuclei further apart.
- Notation:
- An asterisk (σ or π**) indicates that an orbital is antibonding.
3.2 Types of Antibonding Orbitals
- Sigma Antibonding (σ):*
- Occurs when the same atomic orbitals that formed a bonding sigma orbital interact in a way that destroys the bond.
- Pi Antibonding (π):*
- Occurs when p-orbitals overlap laterally, resulting in a node between the nuclei.
4. Energy Levels & Electron Configuration
- The energy levels of bonding and antibonding orbitals must be considered while creating a molecular orbital diagram for a molecule.
- Electrons fill lower-energy bonding orbitals first before moving to higher-energy antibonding orbitals.
- Electron filling follows these principles:
- Aufbau Principle: Fill lower-energy orbitals first.
- Hund’s Rule: Electrons occupy degenerate orbitals singly before pairing.
- Pauli Exclusion Principle: Each orbital can hold only two electrons with opposite spins.
5. The Impact of Bonding and Antibonding Orbitals
- Understanding which molecular orbitals bond and which do not bond is critical for both theoretical and practical chemistry and physics.
- This knowledge helps in:
- Predicting molecular stability.
- Understanding chemical reactivity.
- Designing new materials and drugs.
What's Your Reaction?