Ground State of the Hydrogen Atom Using the Variational Method
The variational method is a powerful technique in quantum mechanics used to approximate the ground-state energy of systems like the hydrogen atom. By assuming a trial wave function and optimizing its parameters, the method estimates energy values close to the true solution of the Schrödinger equation. For the hydrogen atom, using an exponential trial function gives a result equal to the exact ground-state energy, demonstrating the accuracy of the variational approach in predicting electron behavior around the nucleus.
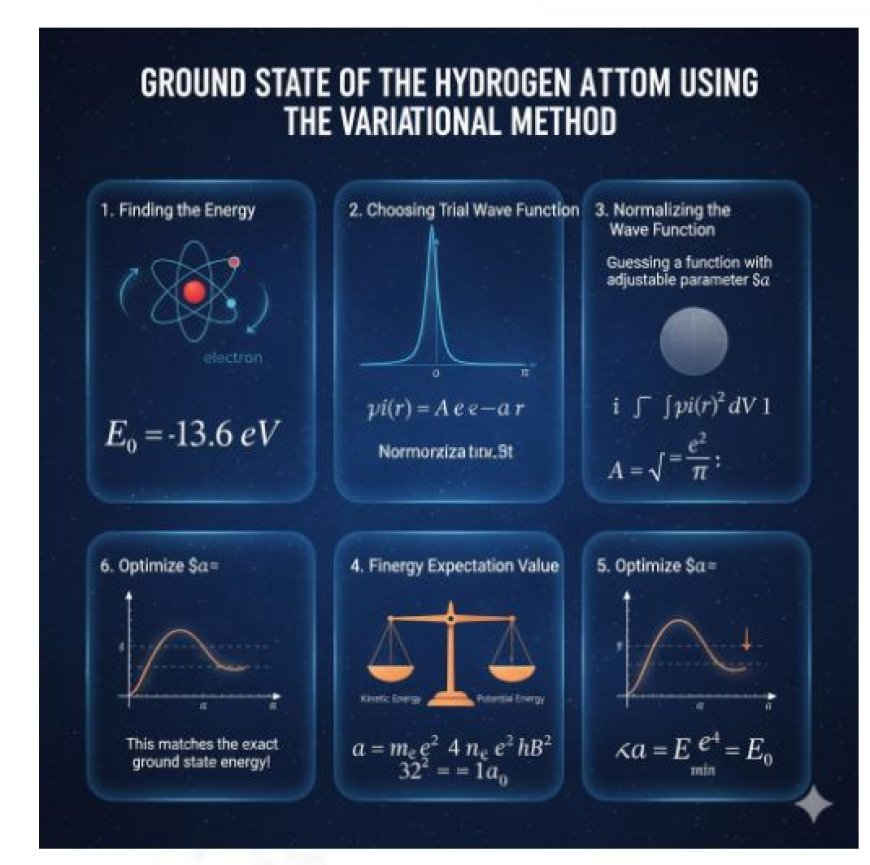
Ground State of the Hydrogen Atom Using the Variational Method
Let’s use the variational method to estimate the ground-state energy of a hydrogen atom.
1. Figuring Out How Much Energy a Hydrogen Atom Has
In quantum mechanics, we find the energy levels of the hydrogen atom by solving the Schrödinger equation for the electron. The exact ground-state energy is:
This is the lowest possible energy an electron can have in a hydrogen atom.
2. Choosing a Trial Wave Function
To apply the variational method, we guess a function that describes how the electron might be distributed around the nucleus. A simple choice is an exponential function:
Here:
- A is a constant that ensures the wave function is normalized.
- α\alphaα is an adjustable parameter that we will optimize to get the best estimate of the energy.
3. Normalization of the Wave Function
To make sure the probability of finding the electron anywhere in space is 1, the wave function must satisfy the condition:
Solving this equation gives us the value of A:
This ensures that our trial wave function is properly scaled.
4. Finding the Energy Expectation Value
Using the variational principle, we calculate the expected energy using our trial wave function. The total energy consists of two parts:
- Kinetic energy, which depends on how fast the electron moves.
- Potential energy, which depends on the attraction between the electron and the nucleus.
After performing the necessary calculations, we get the total energy as:
5. Optimizing α to Minimize Energy
To get the best approximation, we choose the value of α that minimizes the energy. This gives:
where a0 is the Bohr radius, a fundamental length scale in atomic physics.
Substituting this value of α back into the energy equation gives:
which is exactly the true ground-state energy of hydrogen!
What's Your Reaction?