Liquid Drop Model, Shell Model, and Collective Mode
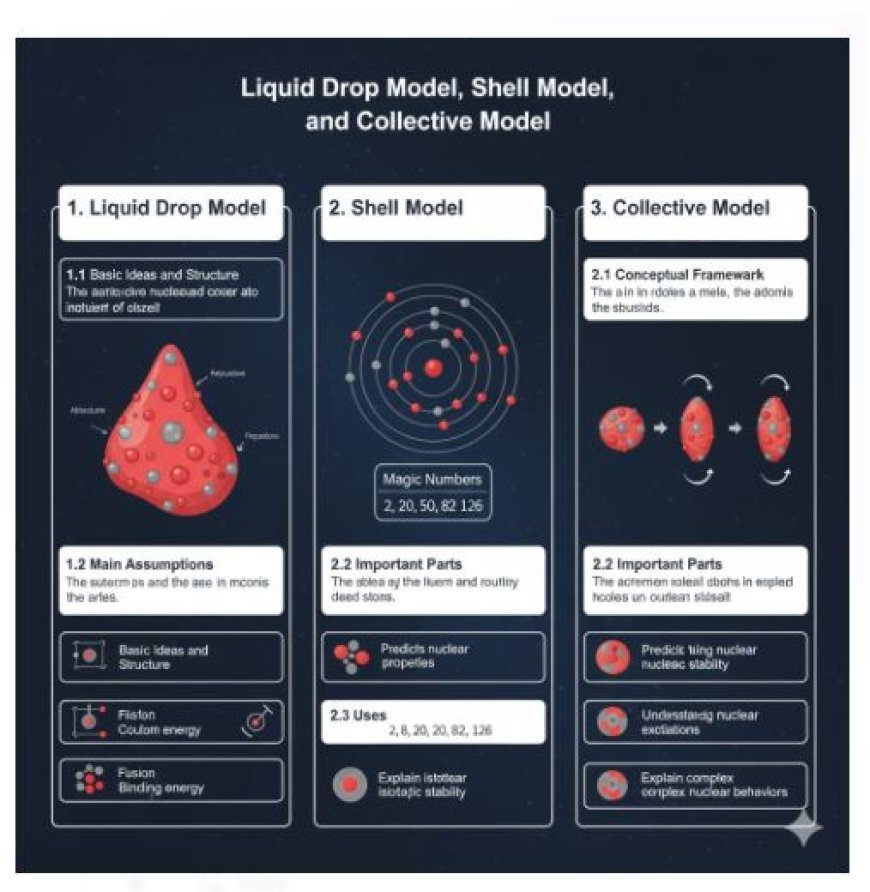
The Liquid Drop Model, Shell Model, and Collective Model are three key frameworks that explain how atomic nuclei behave and remain stable. The Liquid Drop Model treats the nucleus like a droplet of fluid governed by nuclear and Coulomb forces. The Shell Model applies quantum mechanics to describe nucleons arranged in energy levels with “magic numbers.” The Collective Model combines both, explaining vibrations, rotations, and shape deformations in complex nuclei. Together, these models help predict nuclear energy, stability, and reactions.
Liquid Drop Model, Shell Model, and Collective Model
Nuclear physics looks at the parts, structure, and behavior of atomic nuclei. Physicists have created different models to explain and forecast how nuclei behave, with each model providing its own perspective.
1. Liquid Drop Model
The Liquid Drop Model is an early semi-empirical method used to understand the features of atomic nuclei.
1.1 Basic Ideas and Structure
- Comparison with Liquid Drops: The nucleus is compared to a drop of incompressible liquid, where protons and neutrons work together.
- Forces at Play: The model considers the balance between attractive nuclear forces and repulsive Coulomb forces between protons.
1.2 Main Assumptions
- Volume Energy: Depends on the number of nucleons (protons and neutrons) and represents the binding energy from nuclear forces.
- Surface Energy: Nucleons at the surface have fewer binding connections, affecting stability.
- Coulomb Energy: Represents the electrostatic repulsion between protons due to their electric charge.
- Asymmetry Energy: Accounts for the imbalance between protons and neutrons in the nucleus.
1.3 Uses
- Binding Energy Calculations: Helps predict nuclear binding energies and understand stability.
- Nuclear Fission and Fusion: Explains energy release in nuclear reactions.
2. Shell Model
The Shell Model helps us understand nuclear structure using concepts from quantum mechanics.
2.1 Conceptual Framework
- Nucleon Arrangement: Nucleons are arranged in specific energy levels or shells, similar to electrons in atomic orbitals.
- Fermions: Since nucleons are fermions, they obey the Pauli Exclusion Principle, meaning they fill energy levels up to a maximum limit.
2.2 Important Parts
- Magic Numbers: Certain nucleon numbers (2, 8, 20, 28, 50, 82, 126) create exceptionally stable nuclei. These occur when nucleon shells are completely filled.
- Spin and Parity: Each nucleon has a specific spin, and the entire nucleus has rotational momentum and parity states.
2.3 Uses
- Predicting Nuclear Properties: The Shell Model accurately predicts nuclear spins, magnetic properties, and reaction probabilities.
- Explanation of Isotopic Stability: Helps explain why some isotopes are stable, while others undergo radioactive decay.
3. Collective Model
The Collective Model combines elements of both the Liquid Drop Model and Shell Model.
3.1 Conceptual Framework
- Collective Motion: Nucleons move together, similar to liquid drops or crystals, leading to rotations and vibrations within the nucleus.
3.2 Important Parts
- Shape Deformation: Nuclei can change shape, becoming spherical, elongated, or flattened, which affects their energy levels.
- Rotation and Vibration: Nucleons move together in coordinated spinning and vibrating motions, leading to rotational energy bands in certain isotopes.
3.3 Uses
- Understanding Nuclear Excitations: Explains how nuclei become excited and transition between energy states.
- Explaining Complex Nuclear Behaviors: Useful for describing large nuclei, where both shell effects and collective movements are important.
What's Your Reaction?