Dissociation Energy and Dissociation Products
Learn how dissociation energy measures the strength of chemical bonds and how molecules split into atoms, ions, or radicals. This concept helps explain reaction behavior, material stability, and processes in thermodynamics, astronomy, and biology.
Understanding Dissociation Energy and Dissociation Products
- Dissociation is the process in which molecules break apart into smaller fragments, usually atoms, ions, or simpler molecules.
- This phenomenon is fundamental in physics and chemistry, influencing fields such as thermodynamics, atomic physics, and chemical reactions.
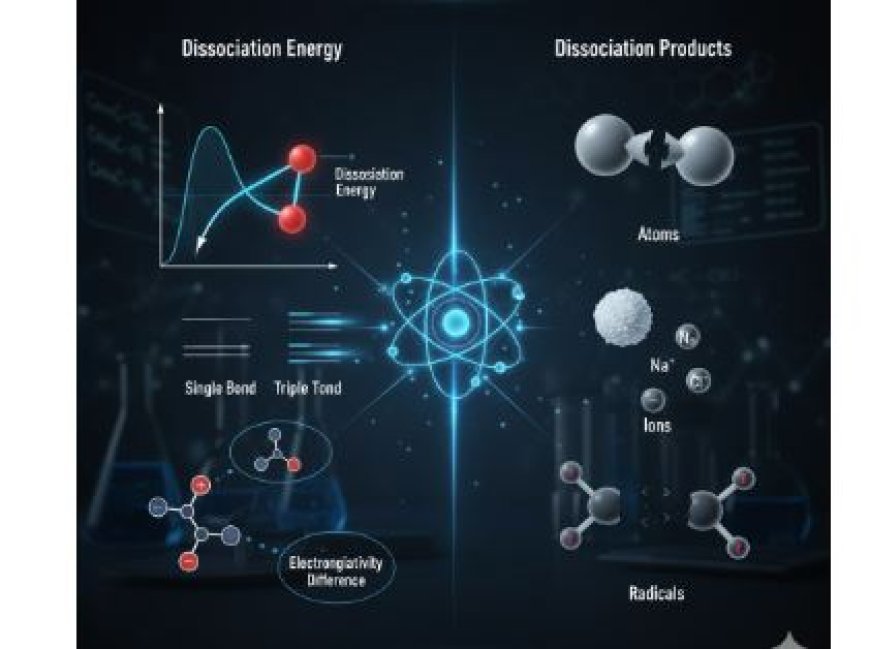
1. Dissociation Energy
- Definition:
- Dissociation energy (or bond dissociation energy) is the amount of energy required to break a specific bond between atoms in a molecule.
- A higher dissociation energy indicates a stronger and more stable bond.
Measurement of Dissociation Energy
- Typically measured in kilojoules per mole (kJ/mol).
- This unit represents the energy needed to break one mole of a specific bond in a substance.
Factors Influencing Dissociation Energy
- Type of Bond:
- Different bond types have different dissociation energies:
- Single bonds → Weakest, lowest energy required.
- Double bonds → Stronger than single bonds, requiring more energy.
- Triple bonds → Strongest, requiring the most energy to break.
- Electronegativity Difference:
- Higher electronegativity differences result in stronger bonds, increasing dissociation energy.
- Molecular Structure:
- The shape of a molecule affects how tightly atoms are bound together, influencing how much energy is needed to break the bonds.
2. Dissociation Products
- Definition:
- Dissociation products are the smaller fragments left behind after a molecule breaks apart.
- These can be atoms, ions, or radicals, depending on the original molecule.
Types of Dissociation Products
- Atoms:
-
- Example: When O₂ dissociates, it forms two oxygen atoms (O).
- Ions:
-
- Some dissociations produce charged particles (ions).
- Example: Sodium chloride (NaCl) dissolves in water, forming Na⁺ and Cl⁻ ions.
- Radicals:
-
- Free radicals are highly reactive species with unpaired electrons.
- Example: The breakdown of hydrogen peroxide (H₂O₂) produces hydroxyl radicals (OH·).
3. Importance of Dissociation Energy and Products
Role in Chemical Reactions
- Understanding dissociation energy helps chemists predict molecular interactions during reactions.
- Reactions involve both bond breaking and bond formation, influencing their feasibility and rate.
Applications in Various Fields
- Thermodynamics:
- Essential in studying heat transfer and energy transformations in chemical processes.
- Materials Science:
- Helps engineers design stronger and more durable materials by understanding how substances break under stress.
- Astronomy:
- Plays a key role in understanding molecular formation and dissociation in stars and interstellar space.
Biological Significance
- In biological systems, dissociation is crucial for:
- Enzyme activity, where substrates bind and release.
- Drug interactions, where molecular bonds determine the effectiveness of medications.
- Understanding these processes aids in drug development and therapeutic advancements.
IMAGE SOURCE (THUMBNAIL)
What's Your Reaction?