THE MAXWELL-BOLTZMANN DISTRIBUTION OF VELOCITY
The Maxwell-Boltzmann distribution: A snapshot of how likely gas molecules are to have different speeds at a given temperature.
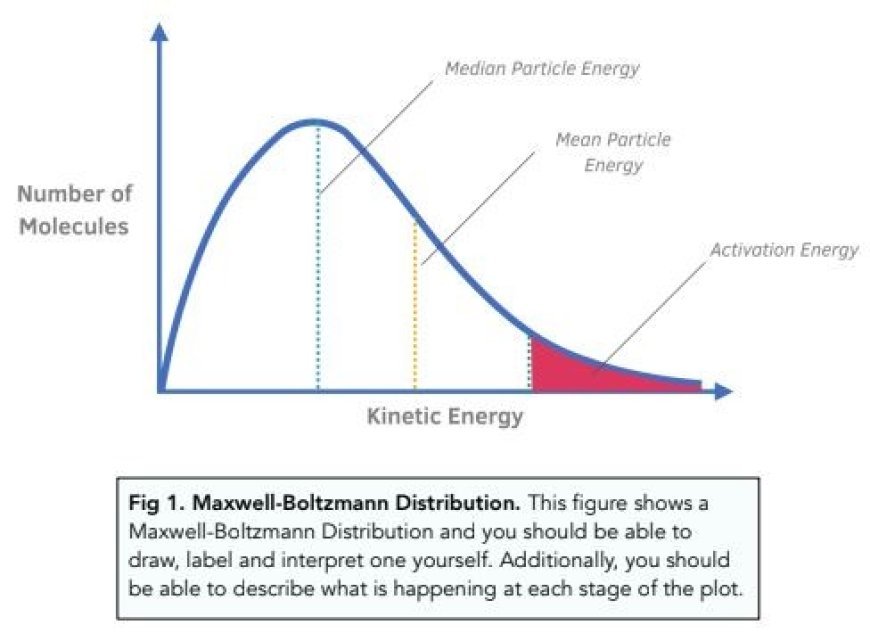
- The Maxwell-Boltzmann distribution, which is sometimes just called the "Maxwell distribution," shows how the energies of molecules in a classical gas are spread out statistically.
- In 1859, the Scottish scientist James Clerk Maxwell was the first person to use probability to explain how the speeds of gas molecules are spread out. In 1871, the German scientist Ludwig Boltzmann built on Maxwell's finding to show how energy is distributed in molecules.
The Maxwell-Boltzmann Distribution of Velocity
- There are huge empty spaces between the thousands of tiny particles (atoms or molecules) that make up gas. All the time, these particles move in every direction.
- When they are moving, they hit each other and the walls of the container. Because of these crashes, the molecules' speed and direction are always changing.
- Because of this, not all the molecules in a sample of gas move at the same speed. The speeds of different molecules are spread out over a large area.
- There is a chance that the particles started moving at the same speed, but when they hit each other, they will change speeds.
- Different molecules are also moving at different speeds. But at a certain temperature, the way the speeds of molecules are spread out stays the same, even though the speeds of individual molecules change.
- Because of this, the number of molecules moving at a certain speed stays the same. We call it the Maxwell-Boltzmann distribution law or just "Maxwell law" because they came up with it first. It is also known as the distribution of speeds.
- They drew a graph with the speeds of molecules (along the x-axis) and the fraction of molecules that move at different speeds (along the y-axis).
- "Maxwell's distribution curve" is the name of the curve that was made.
- Maxwell Distribution of Velocities: The Maxwell distribution of velocities is made up of the following main terms:
- It's very unlikely that there are many molecules moving at very low or very high speeds.
- The number of molecules moving faster rises until it hits a peak, after which it starts to fall. There is a speed for the largest group of molecules, which is the same as the curve's top. This speed is called the most likely speed.
- We can say that the speed of a gas is most likely the speed of the largest group of gas molecules at a certain temperature.
The Effect of Temperature on Speed Distribution
- As the temperature of the gas rises, the chemical motion speeds up.
- The number of the most likely speeds goes up as the temperature goes up. In fact, the whole distribution chart moves to the right as the temperature goes up.
- To put it another way, the slope gets wider as temperatures rise. Although it's important to note that if the temperature stays the same, the speed of molecules doesn't change.
- This is because the mass of the molecules also changes the speed distribution at a certain temperature.
- When the temperature stays the same, molecules of heavy gases move more slowly than molecules of lighter gases.
- For example, molecules of nitrogen move faster than molecules of chlorine, which are heavy.
- With this in mind, nitrogen molecules are more likely to move than chlorine molecules at any given temperature.
Application of the Distribution Law
- The Maxwell-Boltzmann equation, which is the basis of gas kinetic theory, shows how the speeds of gases at a certain temperature are spread out.
- The most likely speed, the average speed, and the root-mean-square speed can all be found with this distribution function.
- The Maxwell-Boltzmann distribution is a type of probability distribution that is used in chemistry and physics.
- The most common use is in the area of statistical mechanics. When molecules and atoms move, they change the temperature of any huge physical system.
What's Your Reaction?