RADIOACTIVITY AND LAW OF RADIOACTIVITY
Nature's Radioactive Engine: Understand how rock decay fuels geological processes and shapes our planet.
Radioactivity
- Due to nuclear instability, radioactivity happens in the center of an atom.
- Radiation that comes from an atom's weak center wastes energy.
- The nucleus stays together because of two forces: the electrostatic force that pushes it away and the strong force that pulls it toward itself.
- In the natural world, these two forces are thought to be very strong.
- As the size of the nucleus grows, so does the chance of volatility.
- This is because the mass of the nucleus gets very concentrated.
- Atoms of Plutonium and Uranium are very fragile because of this, which is why radiation happens.
- Henry Becquerel found out about radium by chance.
- A bundle of uranium compounds wrapped in black paper was put in a box with photography plates.
- When the plates were looked at again later, they were found to be open!
- The idea of radioactive breakdown came from this contact.
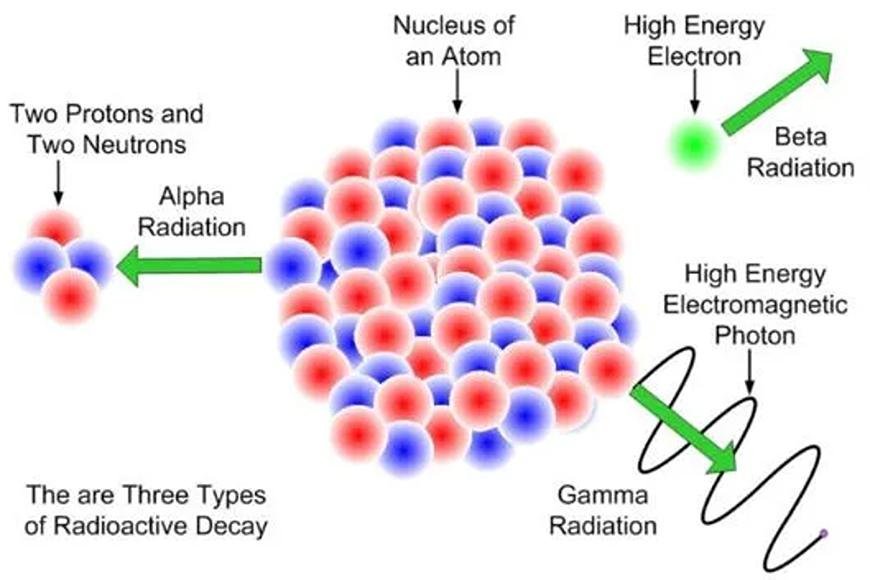
- These are some ways that radioactivity can be seen:
- Photons with a lot of energy are released during gamma decay.
- Electrons are released during beta decay.
- Alpha Decay (emission is made up of helium nuclei).
Laws of Radioactivity
- There is radioactivity when the nucleus breaks down.
- The rate of breakdown of the nucleus is the same no matter what the temperature or pressure is.
- The rule of conservation of charge is important for radioactivity.
- The daughter nucleus is not the same as the mother nucleus in terms of its matter and energy.
- Alpha, beta, and gamma particles are always present when radiation gives off energy.
- The rate at which nuclear materials break down depends on how many atoms are present at the time.
Units of Radioactivity
- In terms of radioactivity, Curie and Rutherford are the units.
- The link between Curie and Rutherford is 1C = 3.7 × 104 Rd.
Uses of Radioactivity
- Here are some ways that radioactivity can be used.
- Americium-241 is an alpha generator that is used in smoke alarms in homes in the US.
- There is a small current in the chamber of the smoke detector because the americium sample gives off alpha particles that ionize the air in it.
- When smoke enters the chamber, the current drops, which sets off the warning.
- Alpha particles have a very short range, but when they get close, they do a lot of damage.
- When alpha emitters are swallowed, they come into close touch with flesh and kill. This is why they are used in attempts to kill people by radiation poisoning.
Advantages and Disadvantages of Radioactivity
Advantages of radioactivity
- Gamma-rays kill cancer cells, which is why they are used in radiotherapy.
- Cobalt-60 is used to kill cells that can cause cancer.
- Gamma rays are used to look inside the body.
- Gamma rays kill bacteria in food, which keeps it from going bad and extends its shelf life.
- Radioactive radiations can be used to figure out how old rocks are by measuring how much argon is in the rock.
Disadvantages of radioactivity
- A lot of radioactive radiation can kill you.
- It costs a lot to buy radioactive isotopes.
What's Your Reaction?