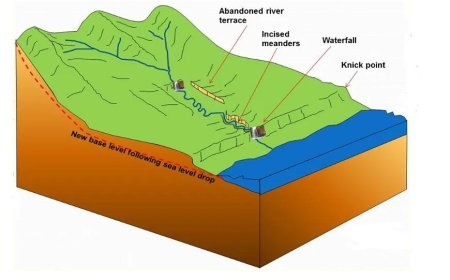
RADIOMETRIC DATING
Radioactivity: Unstable atoms decay, revealing Earth's history through radiometric dating of rocks.
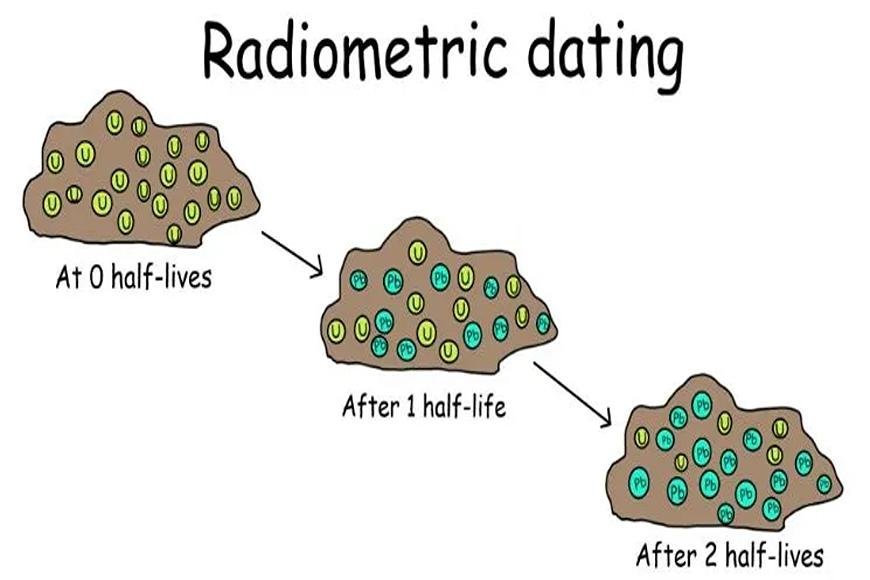
Radiometric Dating
- When radioactive isotopes break down, they give us information about how old rocks, minerals, and skeletons are.
- It is based on the idea that some forms of an element's isotope are unstable and change into more stable forms on their own over time.
- The time it takes for half of the parent isotope to transform into the daughter isotope serves as a measure of how quickly something breaks down.
- Isotopes can be used to date different time periods because their half-lives are different.
- For instance, carbon-14 dating can tell you how old biological things are—up to about 50,000 years old.
- Carbon-14 atoms are taken in and out of the bodies of living things all the time.
- This process stops when it dies, and the carbon-14 that is still there starts to break down.
- Researchers can guess when the entity died by comparing the number of these atoms found in the body to the number that should have been there.
- Carbon-14 has some problems, such as
- Carbon-14 is an unstable form of carbon that is only slightly radioactive. It's used to figure out how old carbon-based things are because their half-life is 5,700 years.
- Because carbon-14 has a half-life of 5,700 years, it can only be used to date things up to 50,000 years old.
Radiometric Dating Methods
- Different nuclear elements that may be in the object are used to figure out how old it is using this method.
There are different kinds of this method.
Potassium-Argon Dating
- The unstable form of potassium breaks down into argon, and the amounts of the two can help figure out how old rocks are.
Uranium-Thorium-Lead Dating
- When uranium, thorium, and lead are used for dating, all of the unstable isotopes of uranium and thorium break down into the steady atom of lead.
- The amounts of these elements in the object can be tested and used to guess how old it is.
What's Your Reaction?