Conservation Laws in Production and Decay Processes
Conservation laws are key principles in physics that ensure certain quantities remain constant during reactions and decays. These include conservation of mass, energy, momentum, charge, baryon number, and lepton number — essential for understanding nuclear and particle interactions.
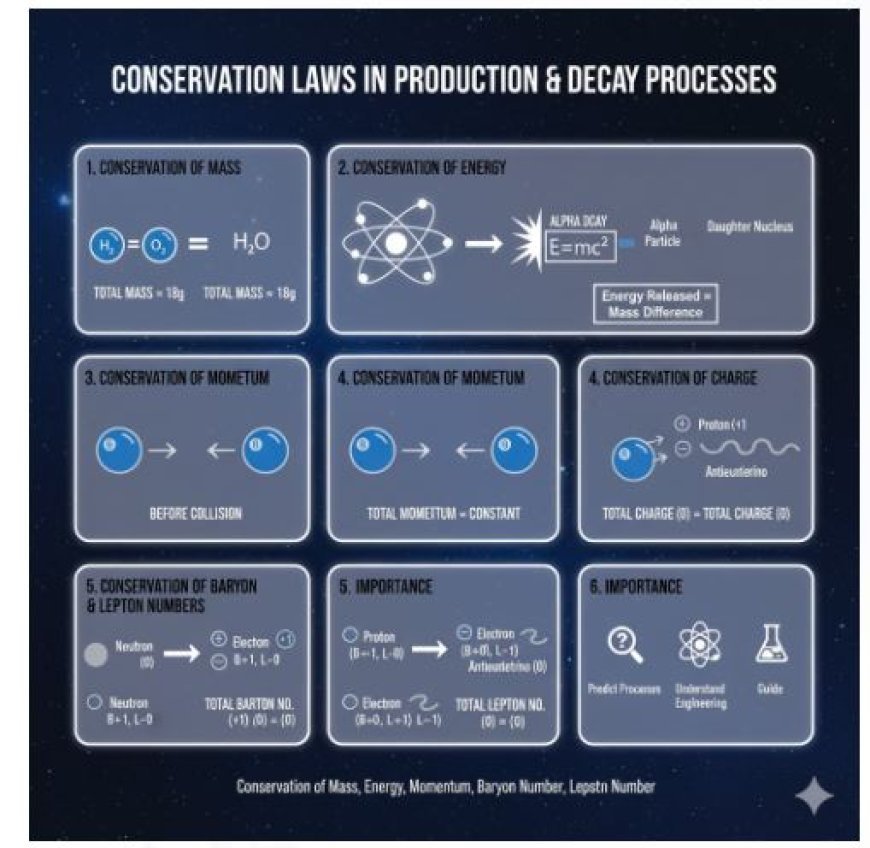
Conservation Laws in Production and Decay Processes
Conservation laws are basic rules in physics that say some features of separate systems stay the same over time. They are important for understanding physical processes, especially how things are made and how they break down.
1. Introduction to Conservation Laws
Conservation rules help us see how various amounts change during physical encounters. They suggest that some measured characteristics stay the same, even as systems change. The main conservation rules are:
- Conservation of Mass
- Conservation of Energy
- Conservation of Momentum
- Conservation of Charge
- Conservation of Baryon Number
- Conservation of Lepton Number
1.1 Why Conservation Laws Matter
Knowing these rules is important for predicting what will happen in physical processes, like nuclear reactions or chemical changes. They act as important rules in areas like physics, chemistry, and engineering.
2. Conservation of Mass
2.1 Definition
The Conservation of Mass says that mass cannot be created or destroyed in a closed system. This concept is important in chemical reactions and nuclear processes.
2.2 Uses in Production and Decay
In any process, the total mass of the starting materials will be the same as the total mass of the final products. For example, if you start with a certain amount of substances in a chemical process, the total mass of the products will be the same as the mass of the starting substances.
2.3 Example
In combustion processes, such as heating hydrogen and oxygen to make water, the total mass of hydrogen and oxygen will equal the mass of the water created.
3. Conservation of Energy
3.1 Definition
The Conservation of Energy means that energy cannot be created or destroyed; it can only change from one type to another.
3.2 Uses in Decay Processes
Energy changes happen during breakdown processes like nuclear decay. In these processes, the energy released must match the difference in mass between what you start with and the products after decay, which is explained by Einstein's equation (E = mc²).
3.3 Example
In alpha decay, a parent atom gives off an alpha particle (which is a helium nucleus) and changes into a new atom called the daughter nucleus. The energy released in this process comes from the mass that is lost during the decay.
4. Momentum Conservation
4.1 Definition
Momentum is what you get when you multiply an object's mass by its speed. The conservation of momentum means that in a closed system, the total momentum stays the same before and after an event.
4.2 Use in Production Processes
In collision processes (such as particle crashes), the total momentum before the collision must equal the total momentum after the collision.
4.3 Example
In nuclear processes like fission, the total momentum of the original nucleus is equal to the total momentum of the fission products and any neutrons that are released.
5. Charge Conservation
5.1 Definition
The Conservation of Charge says that the total electric charge in a closed system stays the same.
5.2 Use in Decay Processes
In nuclear and particle decay, the total electric charge before and after the decay must remain the same.
5.3 Example
When a neutron decays into a proton, an electron, and an electron antineutrino, the overall charge stays balanced before and after the decay. Since the neutron is neutral, and the sum of the products' charges also equals zero, charge is conserved.
6. Conservation of Baryon and Lepton Numbers
6.1 Definitions
- Baryon Number: A measure of how many baryons (like protons and neutrons) are present compared to antibaryons in a system.
- Lepton Number: Like baryons, this counts leptons (like electrons and neutrinos) and their opposite particles.
6.2 Use in Decay Processes
In weak nuclear decays, the total number of baryons and leptons remains the same. This helps scientists understand how different particles can change into one another.
6.3 Example
In beta decay, a neutron changes into a proton and releases a beta particle (electron) and an antineutrino. During this process, the total amount of baryons and leptons stays the same.
What's Your Reaction?