Singlet and Triplet States in the Ground State of Deuteron
This article explores the singlet and triplet quantum states of the deuteron, explaining their spin properties, energy levels, and symmetry differences that define nuclear structure and stability.
Singlet and Triplet States in the Ground State of Deuteron
Introduction to the Deuteron
- A deuteron is a type of atomic particle. It is made up of one proton and one neutron, making it a heavier form of hydrogen. The deuteron is important in nuclear physics and can be found in various nuclear reactions.
- A deuteron is the center of deuterium, which is a type of hydrogen. It consists of one proton and one neutron held together. Studying deuterons is essential in nuclear physics because it helps us understand how more complex atomic nuclei behave.
- When we study the deuteron, we encounter two important quantum states related to the arrangement of its nucleons (proton and neutron): the singlet state and the triplet state.
Understanding Quantum States
- Quantum states are the fundamental way to describe the properties of particles in quantum mechanics. They define everything we can know about a particle, such as its position and momentum.
What are Quantum States?
In science, quantum states represent the different characteristics of a system. Each state includes features like:
- Energy
- Spin
- Rotational motion
For a deuteron, the interactions between its two nucleons can be classified into two key quantum states:
- Singlet state
- Triplet state
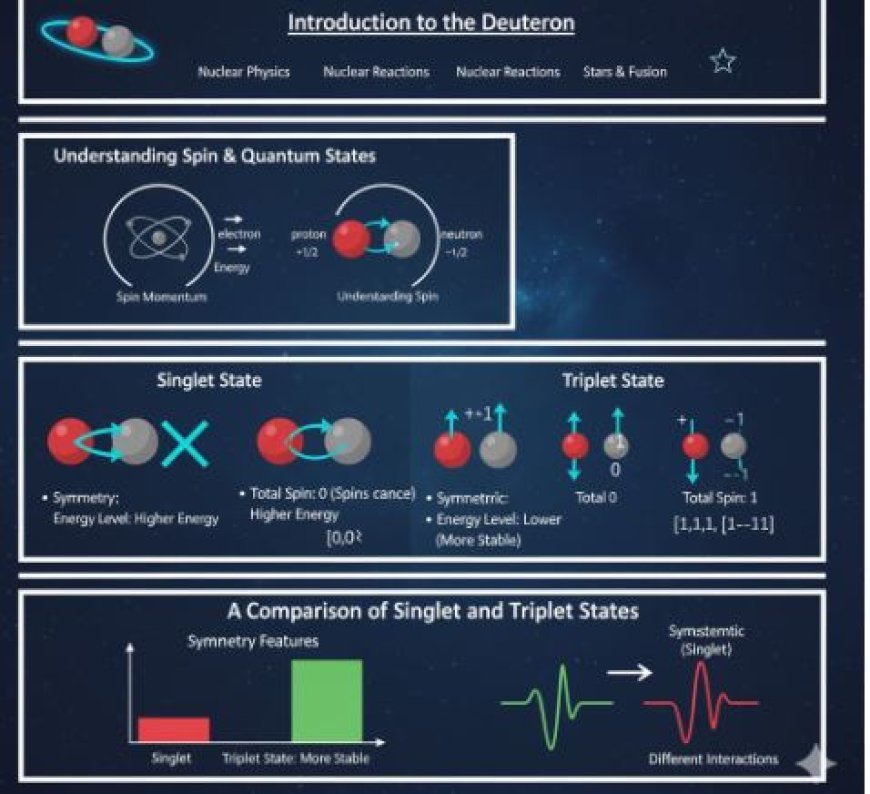
Understanding Spin
Before diving into more detail, it is important to understand spin. Spin is a fundamental type of rotation that subatomic particles possess.
Nucleons (protons and neutrons) can spin in two ways:
- +1/2
- -1/2
The total spin of two interacting nucleons determines whether they are in a singlet state or a triplet state.
Singlet State
In a singlet state, the overall spin of the system is zero. This means that the two nucleons spin in opposite directions, effectively canceling each other out.
In quantum notation, the singlet state is represented as:
∣0,0⟩|0, 0⟩∣0,0⟩
Features of the Singlet State
- Total Spin: The total spin of the singlet state is zero.
- Symmetry: The wave function for a singlet state is antisymmetric. This means that if we swap the two particles, the total function changes direction.
- Energy Level: The singlet state in a deuteron usually has higher energy than the triplet state because of its arrangement.
Triplet State
In a triplet state, the system has a total spin of 1. This corresponds to three different ways the spins of nucleons can be arranged:
- Spin +1: Both spins are pointing up.
- Spin 0: One nucleon has a spin of +1/2 and the other has a spin of -1/2, but they do not completely cancel each other out.
- Spin -1: Both spins are pointing down.
The triplet states are represented as:
∣1,1⟩,∣1,0⟩,∣1,−1⟩|1, 1⟩, |1, 0⟩, |1, -1⟩∣1,1⟩,∣1,0⟩,∣1,−1⟩
Features of the Triplet State
- Total Spin: The total spin of the triplet state is 1.
- Symmetry: The wave function for a triplet state is symmetric. If the two nucleons are swapped, the general effect remains unchanged.
- Energy Level: Triplet states usually have lower energy than singlet states, making them more stable in certain situations.
A Comparison of Singlet and Triplet States
Energy and Stability
- The triplet state is generally more stable and has lower energy than the singlet state.
- This stability explains why deuterons often form in triplet states during nuclear interactions.
Symmetry Features
- The wave function symmetry is crucial in understanding nucleon behavior.
- The singlet state behaves differently from the triplet state due to its antisymmetry, leading to different types of interactions.
What's Your Reaction?