Pure Rotational and Vibrational Raman Spectra of Diatomic Molecules
Explore how Raman spectroscopy reveals pure rotational and vibrational spectra of diatomic molecules through inelastic light scattering. Learn how rotational (ΔJ = ±1) and vibrational (Δv = ±1) transitions produce distinctive spectral lines, enabling identification of molecular structure, bonding, and gas-phase behavior.
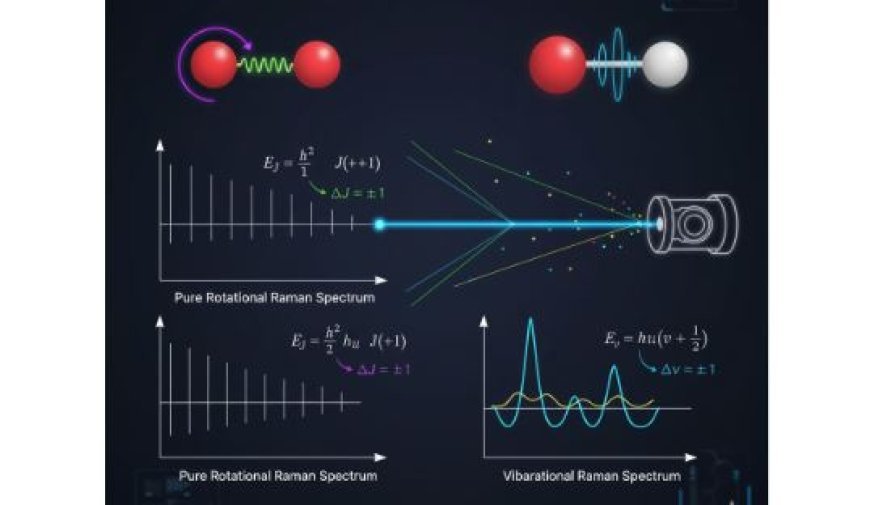
Pure Rotational and Vibrational Raman Spectra of Diatomic Molecules
- In physics and chemistry, Raman spectroscopy is a powerful analytical technique used to study molecular rotations, vibrations, and other low-frequency modes.
- When light interacts with matter, it scatters in ways that reveal molecular structure and motion.
Raman Spectroscopy
- Raman spectroscopy is based on how a monochromatic light source (usually a laser) scatters.
- When light interacts with a molecule, most of it undergoes elastic (Rayleigh) scattering, but a small portion is inelastically scattered, leading to a change in wavelength.
- These wavelength shifts provide insights into the molecular vibrational and rotational energy levels.
Key Components
- Incident Light: A laser emitting a single color.
- Scattered Light: Light that changes direction upon interacting with molecules.
- Detection System: A spectrometer that measures the intensity and wavelengths of the scattered light.
Diatomic Molecules and Their Properties
Why Are Diatomic Molecules Important?
- Diatomic molecules consist of either:
- Homonuclear diatomic molecules (e.g., O₂, N₂).
- Heteronuclear diatomic molecules (e.g., CO, HCl).
- Their simple structure makes their rotational and vibrational energy levels easy to analyze.
Importance in Spectroscopy
- Diatomic molecules are frequently studied because their rotational and vibrational transitions are distinct and well-defined.
- A thorough understanding of these transitions allows scientists to accurately interpret spectral data.
Pure Rotational Raman Spectrum
Definition
- Pure rotational Raman spectra arise when a molecule undergoes a transition between rotational energy levels.
- For diatomic molecules, these transitions occur as the rotational quantum number (J) changes.
Characteristics
- Energy Levels:
The rotational energy levels of a diatomic molecule are given by:
where:
- Ej is the rotational energy.
- h is Planck’s constant.
- I is the moment of inertia.
- J is the rotational quantum number.
- Selection Rules:
- For pure rotational transitions, the selection rule is ΔJ = ±1.
- This means the molecule can transition between adjacent rotational states.
- Appearance in the Spectrum:
- The rotational Raman spectrum consists of evenly spaced sharp lines, corresponding to energy differences between rotational levels (J states).
Applications
- Identification of molecular species using rotational Raman signatures.
- Structural analysis of molecules based on rotational constants.
- Studying gas-phase molecular behavior under varying temperature and pressure conditions.
Vibrational Raman Spectrum
Definition
- Vibrational Raman spectra arise from transitions between vibrational energy levels.
- These transitions involve bond stretching and bending motions within the molecule.
Characteristics
- Energy Levels:
Vibrational energy levels follow the harmonic oscillator model:
where:
- Ev is the vibrational energy.
- v is the vibrational quantum number.
- ν is the vibration frequency.
- Selection Rules:
- For Raman-active modes, the primary selection rule is Δv = ±1, meaning a molecule can transition between adjacent vibrational energy levels.
- Overtones and Combination Bands:
- Overtones (Δv = 2) correspond to higher-order transitions.
- Combination bands involve multiple vibrational modes being excited simultaneously.
Applications
- Determining molecular structure and chemical bonding characteristics.
- Investigating molecular shape changes due to vibrations.
- Analyzing chemical and physical behavior under different conditions.
IMAGE SOURCE (THUMBNAIL)
What's Your Reaction?