Nuclear Magnetic Resonance (NMR)
Discover how Nuclear Magnetic Resonance (NMR) reveals molecular structure through nuclear magnetic properties. Learn its principles, resonance process, and major applications in chemistry, medicine, and scientific research.
Understanding Nuclear Magnetic Resonance (NMR): The Magic of Magnetic Properties of Nuclei
Introduction
- Nuclear Magnetic Resonance (NMR) is a fascinating technique that blends physics and chemistry to study atomic nuclei and their magnetic properties.
- By analyzing how atomic nuclei interact with magnetic fields, scientists can uncover molecular structures and compositions.
NMR
- Definition:
-
- NMR is a technique used to examine the magnetic properties of atomic nuclei.
- It provides valuable insights into molecular structures and is widely used in science, biology, and medicine.
- Key Applications of NMR:
-
- Medical Imaging:
- Forms the basis of Magnetic Resonance Imaging (MRI), allowing doctors to visualize internal body structures.
- Chemistry:
- Helps chemists determine molecular structures and identify compounds.
- Scientific Research:
- Essential for studying DNA, proteins, and complex biological molecules.
- Medical Imaging:
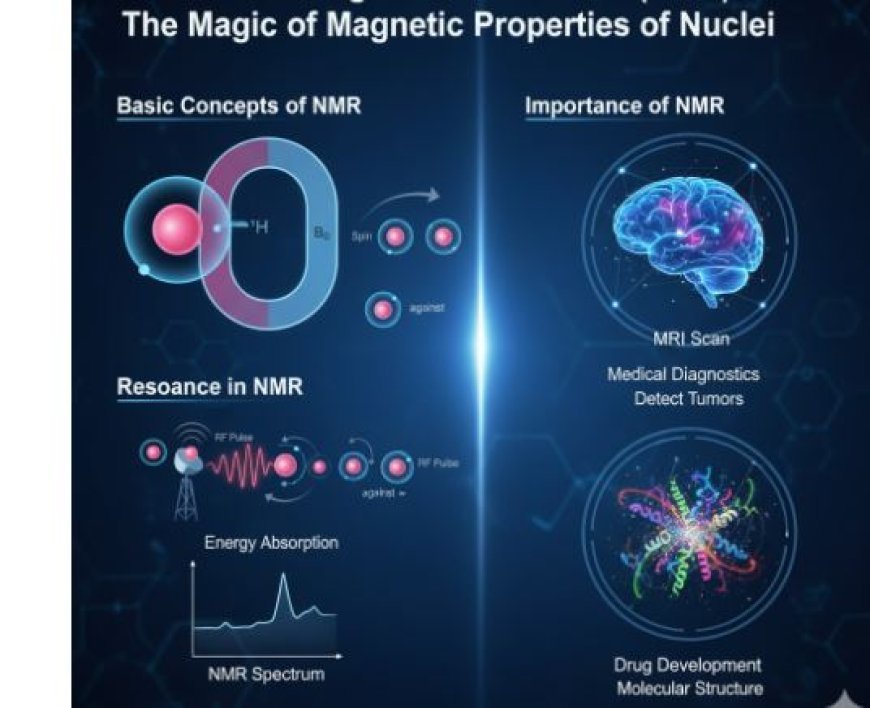
Basic Concepts of NMR
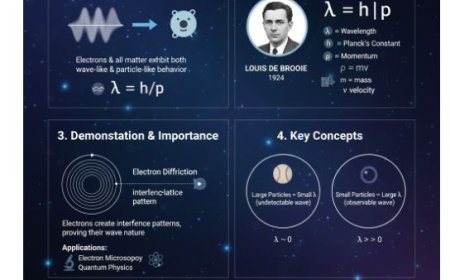
1. Atomic Nuclei and Magnetism
- Atomic nuclei consist of protons and neutrons.
- Certain nuclei, like hydrogen (¹H), possess spin, making them behave like tiny magnets.
- Magnetic Moment:
- Represents the strength and direction of a nucleus’s magnetic field.
- When placed in a magnetic field, nuclei align either with or against the field.
2. Resonance in NMR
How Resonance Works
- Resonance occurs when an external force matches the natural frequency of a system, causing it to oscillate.
- In NMR, this external force is a radiofrequency (RF) pulse.
Steps in NMR Resonance
- Applying a Magnetic Field:
- A strong magnetic field is applied to the sample, aligning the nuclei in specific directions.
- Radiofrequency (RF) Pulse:
- A radiofrequency pulse excites the nuclei, causing them to absorb energy.
- Energy Absorption:
- If the RF pulse matches the nuclei’s resonant frequency, they absorb energy and shift their alignment.
- Relaxation and Signal Detection:
- When the pulse stops, nuclei return to their original state, releasing energy that is measured to create an NMR spectrum.
Key Factors Affecting NMR
- Resonance Frequency:
- Depends on the magnetic field strength and the type of nucleus being studied.
- Chemical Environment:
- Nearby atoms and molecular bonds influence the resonant frequency, helping scientists determine chemical structures.
3. Importance of NMR
Why is NMR Important?
- Non-Destructive:
- The sample remains unchanged, allowing for repeated analysis.
- Highly Accurate:
- Provides precise data about molecular structure, movement, and interactions.
Real-World Applications of NMR
- Medical Diagnostics:
- MRI scans, which use NMR principles, help detect tumors, organ damage, and neurological disorders.
- Drug Development:
- NMR helps analyze drug interactions with biological molecules, aiding in new drug discoveries.
- Chemical and Biological Research:
- Used in organic chemistry, biochemistry, and materials science to determine molecular composition.
IMAGE SOURCE (THUMBNAIL)
What's Your Reaction?