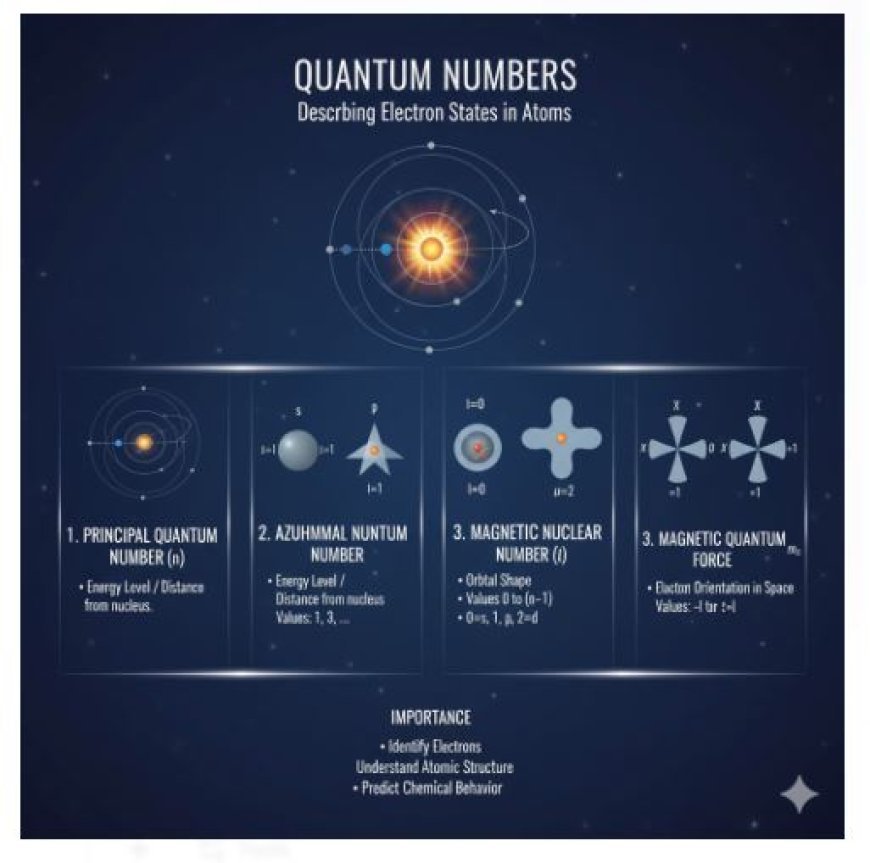
Quantum Numbers
Quantum numbers describe the unique states of electrons in an atom. They define an electron’s energy level, orbital shape, orientation, and spin. Understanding quantum numbers is key to explaining atomic structure, electron configuration, and chemical behavior in quantum mechanics.
Quantum Numbers
- Quantum numbers are used in physics to describe the different states of particles.
- Quantum mechanics is an important theory in physics that explains how matter and energy work at tiny levels, like atoms and small objects. A basic idea in quantum physics is quantum numbers.
- These numbers explain a particle's unique quantum state and give important information about its characteristics.
Quantum numbers are numbers that describe the properties of electrons in an atom. They help identify where an electron is located and how it behaves. There are four main quantum numbers:
- Principal quantum number (n): This number indicates the energy level of an electron and how far it is from the nucleus.
- Angular momentum quantum number (l): This number describes the shape of the electron's path or orbital.
- Magnetic quantum number (m_l): This number shows the orientation of the orbital in space.
- Spin quantum number (m_s): This number indicates the direction of the electron's spin, which can be either up or down.
- Together, these numbers help us understand the arrangement and behavior of electrons in an atom.
- Quantum numbers are numbers that explain the characteristics of particles, especially electrons in atoms.
- They can be seen as "addresses" that provide key information about an electron's energy, shape, and position in its orbital.
Importance of Quantum Numbers
- Identifying Electrons: Quantum numbers help us recognize each electron in an atom.
- Understanding Atomic Structure: They help explain how electrons are arranged and how this affects how atoms behave.
- Predicting Chemical Characteristics: Quantum numbers are important for understanding an atom's chemical characteristics and how it interacts with other atoms.
Types of Quantum Numbers
There are four main quantum numbers that describe an electron's state in an atom:
1. Principal Quantum Number (n)
- Definition: The principal quantum number, or 'n,' shows the main energy level of an electron in an atom.
- Values: Positive whole numbers like 1, 2, 3, and so on.
- Importance:
- Higher values of 'n' mean that electrons are located farther from the nucleus.
- It defines the energy level and size of the orbital, with bigger 'n' values indicating higher energy levels.
2. Azimuthal Quantum Number (l)
- Definition: The azimuthal quantum number, called 'l,' indicates the shape of an electron's orbital.
- Values: Whole numbers from 0 to (n-1).
- If l = 0, the form is a sphere (s orbital).
- If l = 1, the form is a dumbbell (p orbital).
- If l = 2, the form is more complex (d orbital).
- Importance:
- The value of 'l' affects the electron's rotational momentum.
- It helps identify the different orbital shapes.
3. Magnetic Quantum Number (m_l)
- Definition: The magnetic quantum number, called 'm_l,' indicates how the orbital is positioned in space.
- Values: Range from -l to +l, including zero.
- Example: If l = 1 (p orbital), then m_l can be -1, 0, or +1.
- Importance:
- This quantum number shows how many orbitals exist for a specific shape.
- It determines orbital orientation in a magnetic field.
4. Spin Quantum Number (m_s)
- Definition: The spin quantum number, shown as 'm_s,' tells us about the spin nature of an electron.
- Values: +1/2 or -1/2, representing the two possible electron spins.
- Importance:
- Electrons in the same orbital must have different spins, which follows the Pauli Exclusion Principle.
- Spin influences how electrons are arranged in an atom’s orbitals and affects the overall electron configuration.
What's Your Reaction?