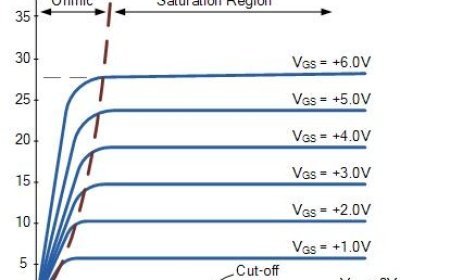
THERMODYNAMIC EQUILIBRIUM
Thermodynamic equilibrium: a state of stable temperature, pressure, and no net flow of matter or energy.
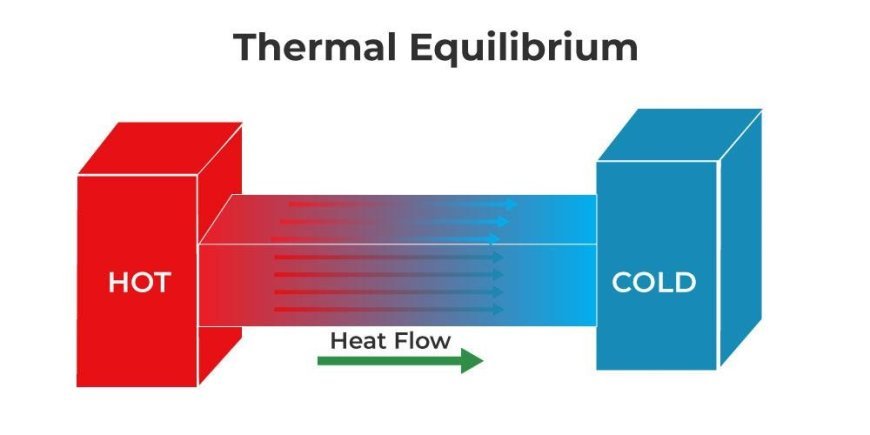
Equilibrium in thermodynamics
- Every part of a system has a set value in a certain state. In this way, the system's state changes if even one property value changes.
- As long as a system is in balance, its traits don't change when it is cut off from its surroundings.
- The system is said to be in thermal balance when the temperature is the same everywhere in it.
- In mechanical balance, the pressure doesn't move anywhere in the system.
- People say that a system is in chemical balance when its chemical make-up doesn't change over time.
- When the masses of two phases hit a state of balance, the system is said to be in phase equilibrium.
- When all three of these conditions are met, a thermodynamic system is in thermodynamic equilibrium. This means that the factors that affect it no longer change over time.
What's Your Reaction?