ENTHALPHY
Enthalpy: Heat Content Plus Pressure & Volume - The Energy Dance of a System.
ENTHALPY
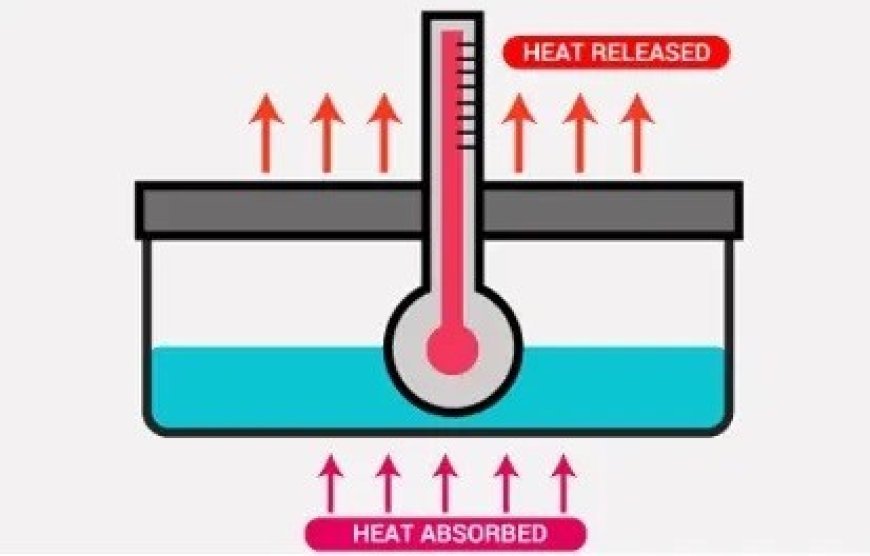
- In a thermodynamic system, enthalpy is a way to measure energy.
- The total amount of heat in a system is equal to its enthalpy, which is equal to its internal energy plus the product of its volume and pressure.
H = E + PV
- H (enthalpy): It encompasses the total energy content of a system, considering both its internal energy and the energy associated with its volume and pressure.
- e (internal energy): This represents the inherent energy of the system at the molecular level, including the kinetic and potential energy of its particles.
- P (pressure) is the force that the system applies to its surroundings or container.
- Volume (v): This represents the area that the system occupies.
- The pv term (pressure-volume work) accounts for the energy exchanged between the system and its surroundings due to changes in volume against pressure.
- When the system expands against a constant pressure, work is done, and the pv term contributes positively to the enthalpy.
- Conversely, when the system is compressed, work is done on it, and the pv term subtracts from the enthalpy.
Knowing this equation, you can:
- Predict the change in enthalpy (ΔH) for a process by considering changes in internal energy and pressure-volume work.
- Analyze the heat flow (q) during a process by relating it to the change in enthalpy using the first law of thermodynamics: q = ΔH + w (w is the total amount of work the machine did).
- Solve thermodynamic problems involving enthalpy, pressure, volume, and internal energy.
What's Your Reaction?