EINSTEIN THEORY OF PHOTOELECTRIC EFFECT
Einstein's photoelectric effect: Light in packets (photons) ejects electrons, not a smooth wave.
EINSTEIN THEORY OF PHOTOELECTRIC EFFECT
- People think that the German physicist Albert Einstein was one of the best thinkers ever.
- He has made important contributions to many areas, such as general relativity, black holes, and the photoelectric effect.
- The Nobel Prize in physics was given to Einstein in 1921 for finding the photoelectric effect.
- Einstein's brilliant and ground-breaking thought about light changed the world. His method of illumination worked well. The very small building blocks of light are called photons.
- The higher energy in these particles is also known as the quantum of radiation. In this way, light is made up of energy packets, also called quantums.
- Photons get their speed and energy from the light source that sends them out. Einstein won the Nobel Prize for finding the photoelectric effect, which was one of the most important things he ever did.
- Einstein was the first person to think that light could be both a wave and a particle. This is what you call the fact that light is both a wave and a particle.
- The idea of wave-particle duality is at the heart of quantum physics and is what led to the creation of solar cells and electron scanners.
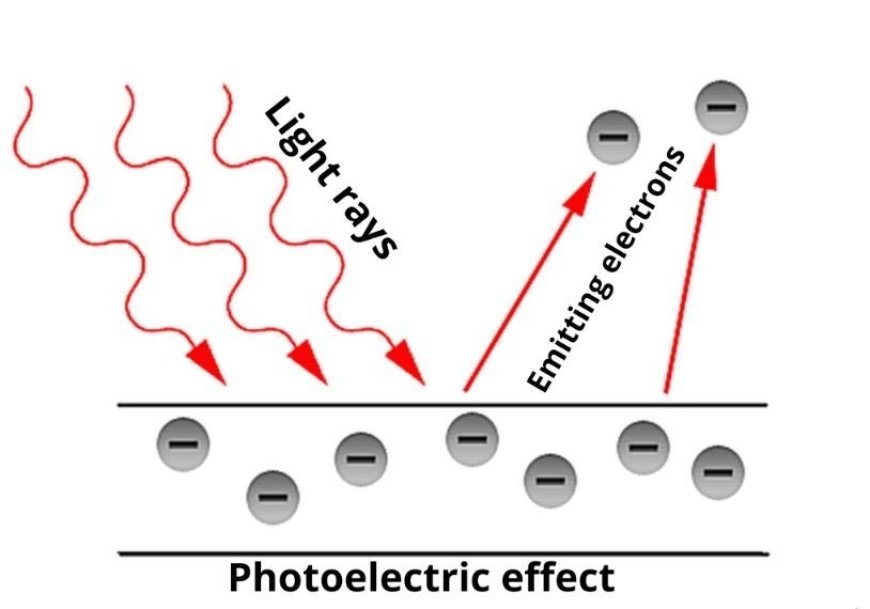
- The photoelectric effect says that when enough energy-rich light hits a metal surface, it makes the metal's electrons fly off. Now, let's try to figure out what the photoelectric effect means.
- His theory of the photoelectric effect says that when light hits a metal surface, the fluctuating electric field makes the electrons inside the atoms gain energy and start to vibrate at a high frequency.
- When the energy of the radiation hitting the metal is higher than its work function, the electrons get enough energy to jump off the surface.
- The speed and number of electrons that are released depend on the radiation's colour, strength, and length of time that it hits the material.
- When there is more radiation hitting something, the electrons receive more energy and move more. This causes more electrons to be sent out at a faster average speed.
- Higher-frequency incident radiation makes the electrons move faster, which makes more electrons escape. In most cases, dim light doesn't give off enough energy for electrons to escape.Einstein's equation for the photoelectric effect is as follows:.
- Planck's quantum theory, which says that light moves in the form of individual photons, served as the basis for Einstein's explanation of the photoelectric effect.
- It is written as hv, where h is a constant and v is the frequency of light.
- E = hv…(1)
- Where "h" is Planck's constant and "ν" is the frequency of the energy being sent out.
- Scientists have tested the photoelectric effect and found that if the radiation hits something with a frequency lower than a certain level, electricity does not flow.
- You can see from the equation that energy is directly linked to frequency. This also explains why electron releases happen right away.
- Since there is no electric field outside the surface, the photoelectron will change into pure motion energy when it leaves the metal.
- The electron partially makes use of the quantum energy that the photons emit in order to overcome the chemical pull of the surface.
- That is, a photoelectron's kinetic energy is equal to the energy it gets from photons minus the energy it uses to leave the surface.
- This energy stays the same on a surface and is shown by. For any given material, this is always the same and is called the work function of a surface.
Thus, the equation is given as K.E. = hν – Φ …(2).
This is Einstein's equation for photoelectricity.
- This is also true for photoelectrons. For the electrons to be thrown off the surface, they need to have the minimum barrier energy.
- Electrons get enough energy to jump off the surface when they are given a cutoff frequency (v0). If the electron gets the same amount of energy as the cutoff frequency, it has no moving energy when it leaves the surface.
With this, we have
(3) Whether hv0 – Φ = 0 or hv0 = Φ
By applying Equation (2) to this, we obtain K.E. = hv – hο0.
or K.E. = h(ν – v0)
Also, v0 is the stopping potential, so
K.E. (max) = eV0, and putting this in equation (3), we have:
The equation eV0 = h (v - v0) (4)
- For the photoelectric effect, this expression is used to find the number of 'h'. This equation gives values that match up with real values, which proves Einstein's theory for the photoelectric effect.
What's Your Reaction?