EINSTEIN THEORY OF PHOTOELECTRIC EFFECT
Einstein's photoelectric effect theory: Light in packets (photons) ejects electrons with energy linked to frequency, not just intensity.
Introduction to Einstein’s Explanation of the Photoelectric Effect
- People think that the German physicist Albert Einstein was one of the best thinkers ever.
- He has made important contributions to many areas, such as general relativity, black holes, and the photoelectric effect.
- The Nobel Prize in physics was given to Einstein in 1921 for finding the photoelectric effect. Einstein's brilliant and ground-breaking thought about light changed the world. His method of illumination worked well.
- The very small building blocks of light are called photons. The higher energy in these particles is also known as the quantum of radiation. In this way, light is made up of energy packets, also called quantums.
- Photons get their speed and energy from the light source that sends them out.
- Einstein won the Nobel Prize for finding the photoelectric effect, which was one of the most important things he ever did. Einstein thought light to be both a wave and a particle for the first time.
- The fact that light is both waves and particles is called this. The idea of wave-particle duality is at the heart of quantum physics and is what led to the creation of solar cells and electron scanners.
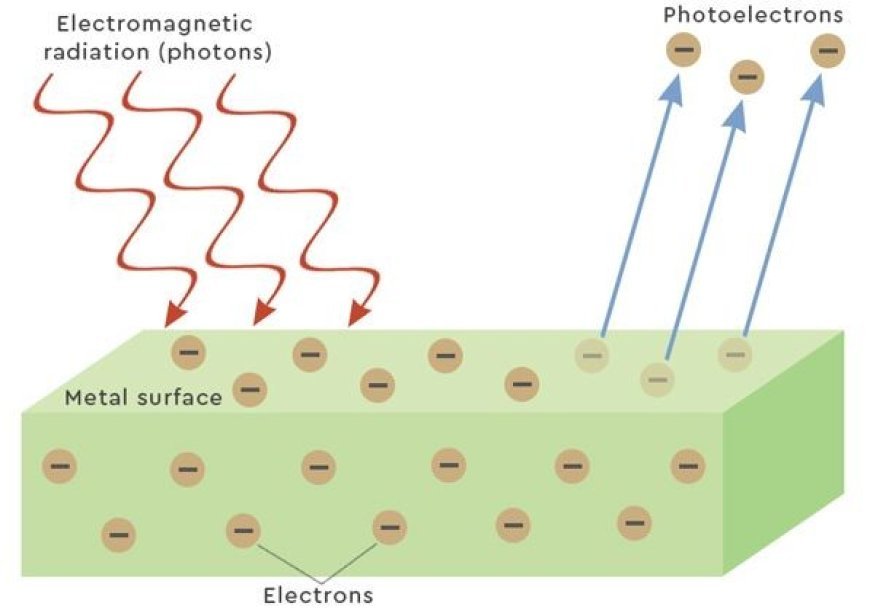
- The photoelectric effect says that when enough energy-rich light hits a metal surface, it makes the metal's electrons fly off. Now, let's try to figure out what the photoelectric effect means.
Einstein's Idea About the Photoelectric Effect
- The fluctuating electric field of the light hits the metal surface, giving the electrons inside the atoms more energy and making them vibrate at a high frequency.
- When the energy of the radiation hitting the metal is higher than its work function, the electrons get enough energy to jump off the surface.
- The speed and number of electrons that are released depend on the radiation's color, strength, and length of time that it hits the material. When there is more radiation hitting something, the electrons receive more energy and move more. This causes more electrons to be sent out at a faster average speed.
- Higher-frequency incident radiation makes the electrons move faster, which makes more electrons escape. In most cases, dim light doesn't give off enough energy for electrons to escape.
Photoelectric Effect
- When light with an energy level higher than the metal's threshold value hits the surface, it frees the metal's tightly bound electrons.
- A "photo" is a tiny bit of light. When a photon hits an electron, it gives the electron all of its energy, which pushes the electron off the surface.
- The photon's extra energy turns into a free negative charge known as a photoelectron.
Einstein's explanation of how the photoelectric effect works
- It is the amount of energy that affects how strong the photoelectric current is, and it should be higher than the cutoff frequency.
- The photocurrent stop was the opposite stopping possibility. It doesn't matter how strong the radiation is that hits it.
- If the frequency of the light that hits the semiconductor is less than the threshold frequency, there is no photoelectric current.
- If you put a metal strip in the sun or light, it won't have the photoelectric effect unless the frequency is higher than the baseline number.
- The photoelectric reaction takes place right away. The metal's electrons come out as soon as light hits the surface.
Planck’s Theory and the Photoelectric Effect
- According to Einstein, Planck's theory was expanded in 1905 to include the photoelectric effect. This is the process by which metal releases electrons when it is exposed to light or strong photons.
- For some metals, electrons don't get released below a certain frequency (0). This is because the kinetic energy of the released electrons depends on the frequency of the radiation, not its strength. Also, fumes start as soon as the light hits; there doesn't seem to be any pause.
- Einstein said that these effects can be explained by two ideas: light is made up of particles called photons, and Planck's equation tells us how powerful each photon is; and a single metal atom can take in a whole photon or anything else.
- Albert Einstein thought that light beats weren't waves traveling through the atmosphere but rather huge groups of different energies. These were called photons because low-frequency beams couldn't make enough energy to make photoelectrons, which are made of light energy from continuous waves.
- The frequency of light is made up of photons in a light wave that have different amounts of energy.
If the electron inside an active material takes in more energy from the photons than it can hold on to, it is more likely to be freed from the imaging process. - A photon with too little energy can't make an electron come out of something.
- Increasing the brightness of low-intensity light will only make more low-energy photons, so there won't be a single photon with enough electron output.
- Additionally, only the energy of the individual photons, not the intensity of the light arriving at a particular frequency, affects the release of electron energy.
- Photoemission can happen in anything, but it happens most often in metals and other conductors. This is because the process creates an imbalance in costs, which makes the barrier grow until the discharge is fully used up.
- If the current flow does not measure this imbalance, it will still occur. As a result of non-conductive oxide ports on metal surfaces making it harder to retrieve images, a lot of practical studies and devices that use the effect of electric photography need tubes with clean metal surfaces.
- The vacuum also makes electron imaging easier because it keeps gases from blocking the flow of electrons between electrodes.
Einstein’s Equation of the Photoelectric Effect
- The Einstein-Planck relation says that Einstein described the photoelectric effect using Planck's quantum theory, which says that light can move in the form of separate photons. It is written as hv, where h is a constant and v is the frequency of light.
E = hv (1)
- Where "h" is Planck's constant and "ν" is the frequency of the energy being sent out.
- Scientists have tested the photoelectric effect and found that if the radiation hits something with a frequency lower than a certain level, electricity does not flow.
- You can see from the equation that energy is directly linked to frequency. This also explains why electron releases happen right away.
- Since there is no electric field outside the surface, the photoelectron will change into pure motion energy when it leaves the metal.
- The electron partially makes use of the quantum energy that the photons emit in order to overcome the chemical pull of the surface.That is, a photoelectron's kinetic energy is equal to the energy it gets from photons minus the energy it uses to leave the surface.
- This energy stays the same on a surface and is shown by. For any given material, this is always the same and is called the work function of a surface.
From this, we can see that
K.E. = hº –...(2)
This is Einstein's equation for photoelectricity.
- This is also true for photoelectrons. For the electrons to be thrown off the surface, they need to have the minimum barrier energy.
- Electrons get enough energy to jump off the surface when they are given a cutoff frequency (v0). If the electron gets the same amount of energy as the cutoff frequency, it has no moving energy when it leaves the surface. Using this, we have
Whether hv0 – Φ = 0 or hv0 = Φ..(3)
Using this in equation (2), we get K.E. = hν – hν0 or
K.E. = h(ν – v0)
v0 is also the stopping potential, so the work of K. (max) = eV0.
Putting this into equation (3) gives us:
eV0 = h (½ - v0)…(4)
- This expression is used to figure out the number of 'h' for the photoelectric effect.
- This equation gives values that match up with real values, which proves Einstein's theory for the photoelectric effect.
Photoelectric effect function
- Einstein's theory of electric shock came from Max Planck's idea that light is made up of very small energy packets called photons or luminous quanta.
- He came up with this idea in 1905. Each file has a power level hv that is equal to the frequency v of the electromagnetic wave that goes with it.
- The Planck constant is named after the h-constant for ratio. When Niels Bohr of Denmark applied quantum theory to the spectra of atoms in 1913, he made a big step forward in this field.
- The range of light that gas atoms give off has been studied in great detail since the middle of the 1800s. It was found that light from gaseous atoms had a number of different bands when the pressure was low.
- Rays from objects, on the other hand, spread out over a wide range of bands. A line spectrum is a set of clearly defined wavelengths that gas atoms give off. This is because the light they give off is made up of a number of straight lines. At the end of the 1800s, experimental scientists had a big problem to solve.
- A scientist named James Clerk Maxwell put out his theory of electromagnetism in 1865.
- It is said that both electricity and the magnetic field move through space like light waves.
- Afterwards, the inspectors went out to get solid proof of Maxwell's ideas, which, at least in theory, correctly explained how light works.
The effect of electromagnetic waves
- The structure of the solid electronic band, also known as the electric conditions' power and pressure distribution, determines the electronic properties of the ordered, shiny solid materials.
- According to the theory of solid-state photoemission, electromagnetic effects are probably what keep this pattern in place.
- There are many different kinds of materials in the electrical world, and the internal photoelectric effect is a direct optical transition between them. This is no longer the case, thanks to quantum mechanical selection rules for dipole moments.
- There is a chance that a second electron release, or Auger effect, can come from a hole that the electron leaves behind, even if the first photoelectron doesn't do this. This process pushes the pens toward solid-molecule targets. These targets can be seen as satellite lines in the electron reserve.
- At the top, electrons are scattering, and other electrons are spreading out because they are interacting with other solid elements.
- Deep inside the material, electrons are more likely to hit each other and come out moving at different speeds.
- The energy of the electrons determines a slope for their non-mechanical process.
What's Your Reaction?