PHASE TRANSITION FIRST AND SECOND ORDER
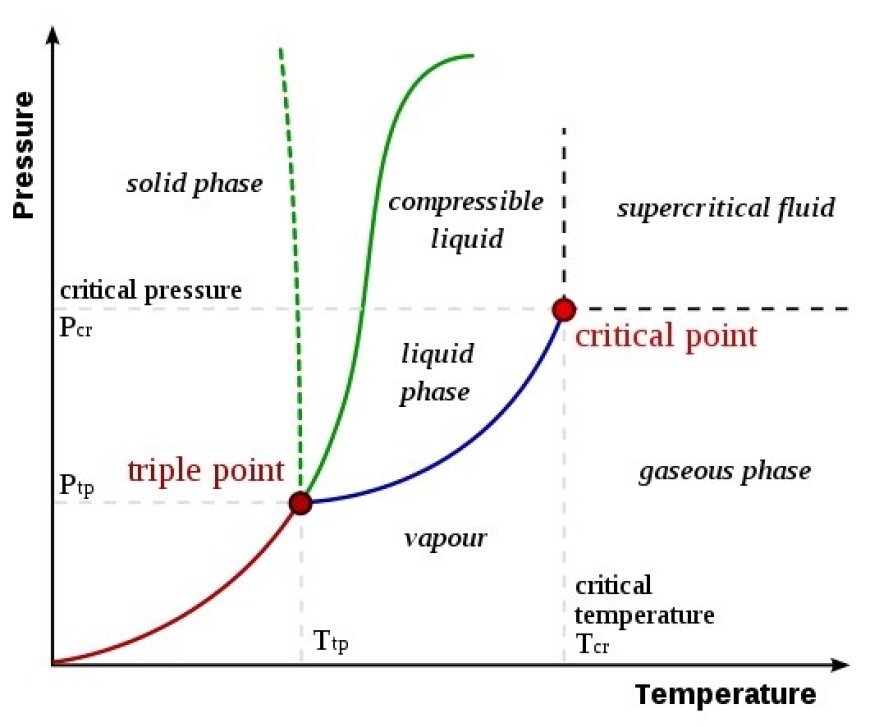
Phase transitions: Big changes (order to disorder or vice versa) happen smoothly (second order) or abruptly (first order) with a jump.
Phase Transition
- In chemistry, thermodynamics, and many other fields, things move from one state to another with different values of certain factors. This is called a phase shift or phase change.
- A "change of state" can happen in any of the three basic states of matter: solid, liquid, or gas. In some rare cases, it can also happen in plasma.
- The physical properties of a phase in a thermodynamic system are the same as the physical properties of a state of matter.
- Change of phase: Some of the things that make up a medium can change quickly, like its temperature, pressure, or other qualities.
- These kinds of changes in the outside world cause this to happen.
- As an example, when a liquid gets hot enough to boil, it might turn into a gas, which changes its volume a lot right away.
- We measure the outside conditions under which the change happens. This is referred to as a phase transition.
- In nature, phase changes happen all the time, and new tools use them all the time.
First-order phase transition
- Phase changes of the first order happen when there is a hidden heat movement.
- An energy shift happens when a system either takes in or gives off a steady, usually large amount of energy per unit measure of the system.
- During this process, the system's temperature will stay the same even if more heat is added; it is in a "mixed-phase regime," where some parts are in a state of transition and other parts are not.
- This is well-known to happen when ice melts or water boils (the water doesn't quickly turn into vapor; it makes a turbulent mixture of liquid water and vapor bubbles).
- Yoseph Imry and Michael Wortis have shown that a first-order shift can become wider when chaos is quenched. This means that the change is finished within a certain temperature range, but things like supercooling and superheating still happen, and hysteresis is seen during the thermal cycle process.
Second-order phase transition
- Continuous phase transitions are another name for second-order phase transitions.
- They happen over time, which is why they are called that.
- There is no end to the length of their links, and when they hit criticality, their correlations drop off in a power law-like way.
- There are many examples of second-order phase changes, such as the ferromagnetic transition, the superconducting transition, and the superfluid transition.
- A Type-I superconductor has a second-order phase transition when there is no external field.
- A Type-III superconductor has a second-order phase transition for both normal-state to mixed-state and mixed-state to superconducting-state.
- The thermal expansion and heat capacity of amorphous materials change more quickly at the glass transition temperature than viscosity. This makes it easier to see with differential scanning calorimetry.
- Lev Landau had an idea about second-order phase shifts.
- There are more than just easy phase changes that can happen on their own.
- Transformation lines and multicritical points show up when you change the magnetic field or the make-up of something.
- A lot of changes happen over and over, which is why they are called "infinite-order phase transitions."
- A lot of people know about the Kosterlitz-Thouless shift in the XY model, but there are a lot more.
- In this group, there are a lot of quantum phase changes, like those that happen in two-dimensional electron gas.
- For people who work with plastics and other products that can be cooled way below their melting point, this change can be seen.
- In many ways, this is not like other things that happen. It's not a change in the thermodynamic ground state in this case.
- A lot of people believe that the real ground state is always crystallized.
- Glass may have different densities, entropies, and other qualities than it would have if it hadn't been heated at all. This is because of how it was fired. This means that the change from liquid to glass is mostly a process: as a liquid cools, more and more of its internal degrees of freedom become out of balance.
- Theories use the idea that if rest times go on forever, there will be a change in the underlying phase.
What's Your Reaction?