FERMI OR FREE ELECTRON GAS
Fermi gas (or free electron gas): Metals as a party - electrons move freely, creating a "sea" with properties explained by quantum statistics.
The Fermi gas
- A Fermi gas, also known as a free electron gas, is a group of fermions that do not interact with one another. For fermionic particles, it is the quantum mechanical equivalent of an ideal gas.
- Fermi gases include electrons in metals and semiconductors, as well as neutrons in a neutron star.
- Based on Fermi-Dirac statistics, the set of available energy levels, the density of the fermions in a Fermi gas, and their temperature determine how their energy is distributed.
- The Pauli principle says that a quantum state can't have more than one fermion. This means that the total energy of the Fermi gas at room temperature is higher than the sum of the single-particle ground state energy and the number of particles.
- As a result, unlike a conventional ideal gas, the pressure of a Fermi gas is nonzero even at zero temperature. This degeneracy pressure holds a neutron star (a Fermi gas of neutrons) or a white dwarf star (a Fermi gas of electrons) together against the inward pull of gravity.
- A Fermi temperature below which the gas is regarded as degenerate can be defined. The energy density of the states as well as the mass of the fermions determine this temperature.
- Metals' electron gas's Fermi temperature is often many thousands of kelvins, indicating that they are degenerate.
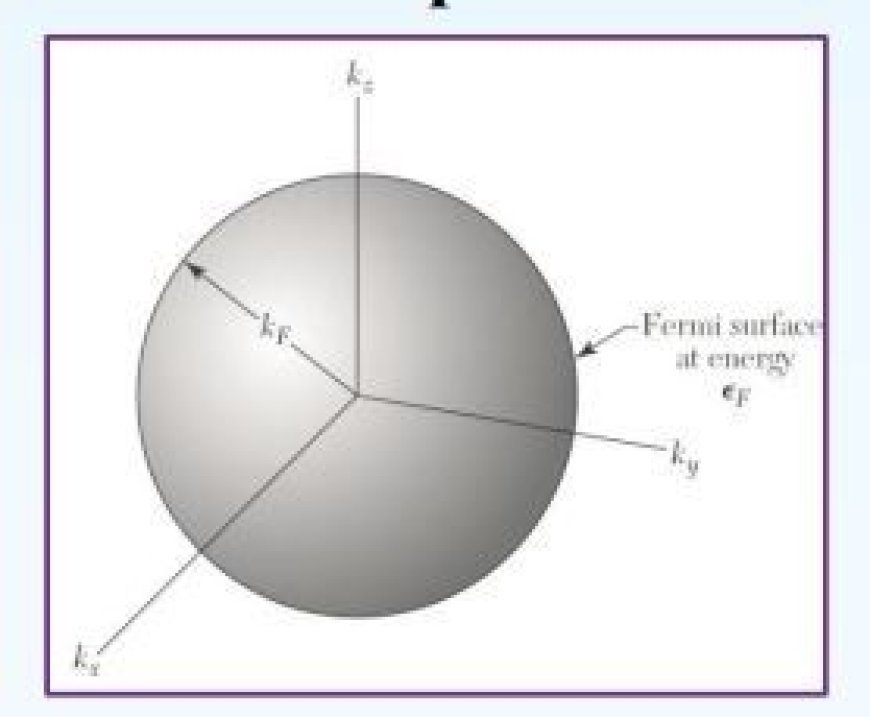
- Fermi energy is the maximal energy of fermions at zero temperature. The Fermi surface is the Fermi energy surface in momentum space.
- Studying the equilibrium characteristics and dynamical behaviour of a Fermi gas comes down to looking at how single particles behave because interactions are, by definition, not taken into account.
- As such, it is still quite tractable and serves as the foundation for more sophisticated theories (such as Fermi liquid theory or perturbation theory in the interaction) that take interactions into consideration to some extent.
What's Your Reaction?